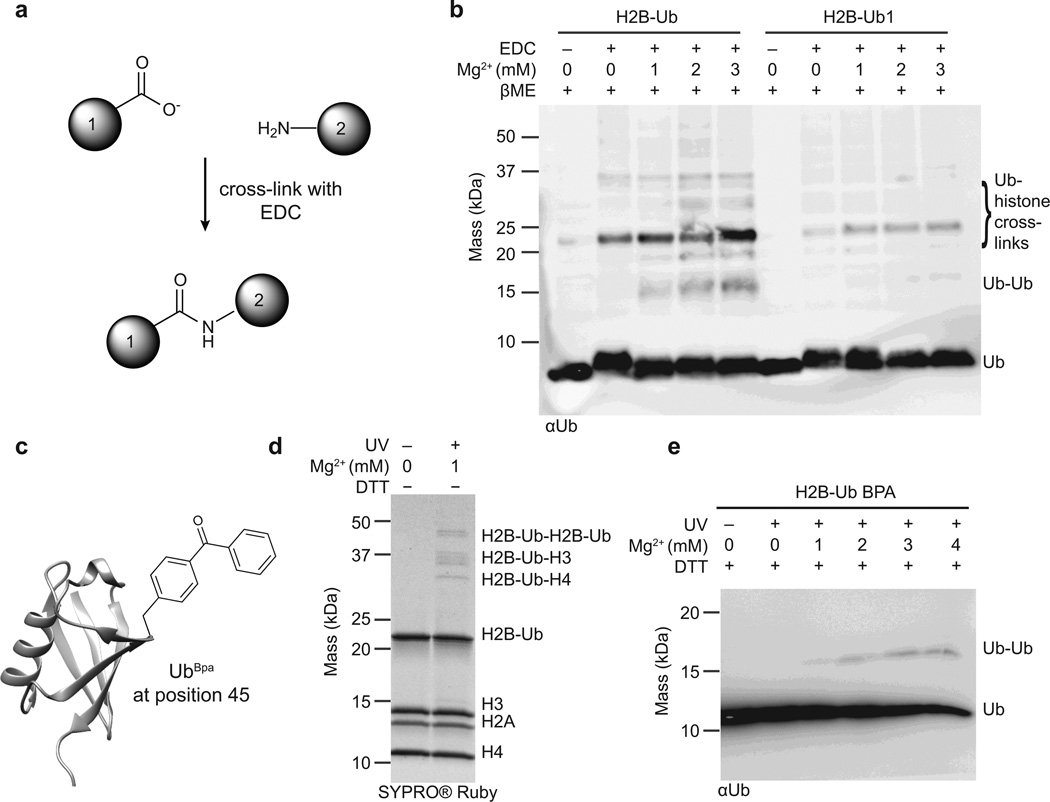
Figure 4.
Ubiquitin-chromatin interactions probed with cross-linking experiments. (a) The zero-length cross-linker EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) couples carboxyl groups to primary amines to form a stable amide bond. (b) EDC-based cross-linking of nucleosome arrays containing H2B-Ub and H2B-Ub1. Cross-linking was performed in the presence of increasing concentrations of Mg2+, and all samples were subsequently reduced with β-mercaptoethanol to detach ubiquitin and ubiquitin cross-linked species from H2B. Analysis was performed by SDS-PAGE followed by western blotting with an antibody against ubiquitin. (c) The site-specific cross-linker Bpa (p-benzoyl-phenylananine) was installed at position 45 on ubiquitin using amber codon suppression. (d) UV-induced cross-linking of H2B-UbBpa arrays in the presence of 1 mM Mg2+. The cross-linked bands were resolved by SDS-PAGE under non-reducing conditions. (e) UV-induced cross-linking of H2B-UbBpa arrays in the presence of increasing concentrations of Mg2+. Samples were reduced with 100 mM dithiothreitol, separated by SDS-PAGE, and analyzed by western blotting against ubiquitin. Representative data from three independent experiments are shown and full images are presented in Supplementary Fig. 15.