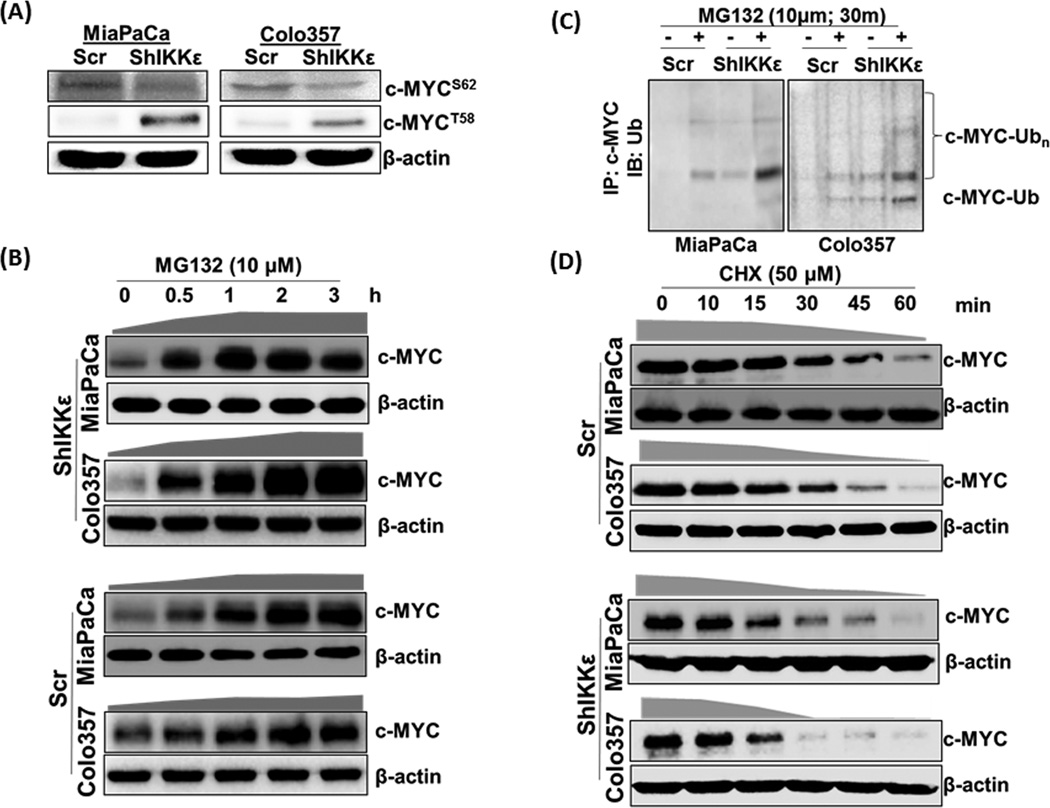
Figure 4. IKKε-induced c-MYC expression and localization is controlled through its inhibition of phosphorylation-mediated nuclear-export and subsequent degradation.
(A) Phosphorylated c-MYCS62 and c-MYCT58 were analyzed by immunoblot using specific-antibodies. (B) Cells were treated with the proteasome inhibitor (MG132, 10µM) for indicated time period, total protein isolated and effect on c-MYC expression determined by immunoblot analysis. We used 60 and 25µg protein from IKKε-silenced and control cells, respectively, to correct for differences in initial protein levels. β-actin was used as loading control. (C) Equal amount (500µg) of protein from Scr and ShIKKε PDAC cells untreated or treated with MG132 (10µM, 30 min) was subjected to immunoprecipitation with anti-c-MYC antibody followed by immunoblot with anti-Ub antibody. (D) To monitor the turnover of c-MYC protein, cells were treated with cycloheximide (50µM), neo-protein-synthesis inhibitor, for indicated time-intervals. Thereafter, total protein was isolated and changes in c-MYC expression monitored by immunoblotting. Considering differences in c-MYC levels between control and IKKε-silenced cells, we used different amounts (60µg and 150µg, respectively) to keep initial signal at near-similar intensity.  - represents the rate of total c-MYC accumulation, and
- represents the rate of total c-MYC accumulation, and  - represents the rate of c-MYC degradation based on densitometry of the data presented.
- represents the rate of c-MYC degradation based on densitometry of the data presented.