Figure 1.
HPCs Isolated from the Chronically Injured Adult Mouse Liver Have Trilineage Differentiation Potential
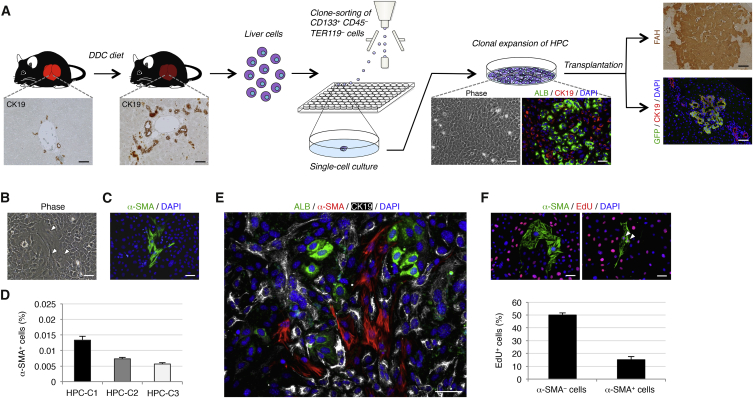
(A) Experimental procedure to isolate and characterize HPCs. Wild-type mice were administered DDC for induction of CK19+ biliary lineage cells that contain a fraction of HPCs in the chronically injured liver. The liver tissues were then dissociated into single cells, and the CD133+CD45−TER119− biliary lineage cells were isolated by flow cytometry and clonally cultured in 96-well plates. HPCs that formed LCs and expanded in clonal culture exhibited the features of epithelial cells and produced hepatocytes and cholangiocytes as descendants, while maintaining undifferentiated cells by undergoing self-renewing cell divisions. Upon transplantation, HPCs marked by expression of GFP were also capable of reconstituting the hepatic lobule as FAH+ hepatocytes and forming the biliary ductal structures by differentiating into CK19+ cholangiocytes. We chose three independent HPC clones for examination.
(B) In clonal cultures of HPCs, a small number of cells with the morphology of mesenchymal cells were present (arrowheads).
(C) Immunofluorescence staining of α-SMA was conducted for cells in a clonal culture of HPCs.
(D) The percentages of cells immunoreactive for α-SMA among HPC clones (HPC-C1, HPC-C2, and HPC-C3) were calculated after counting ∼1 × 105 cells in individual culture dishes.
(E) Co-immunofluorescence staining of ALB with α-SMA and CK19 was conducted for cells in a clonal culture of HPCs.
(F) Co-immunofluorescence staining of α-SMA with EdU was conducted for cells in a clonal culture of HPCs (arrowheads: α-SMA+ EdU+ cells), and the percentages of cells immunoreactive for EdU in α-SMA− or α-SMA+ cells were calculated after counting ∼800 or ∼20 cells, respectively, in individual culture dishes. The data represent means ± SD of three technical replicates (n = 3). DNA was stained with DAPI.
Scale bars, 50 μm. See also Figures S1 and S2.