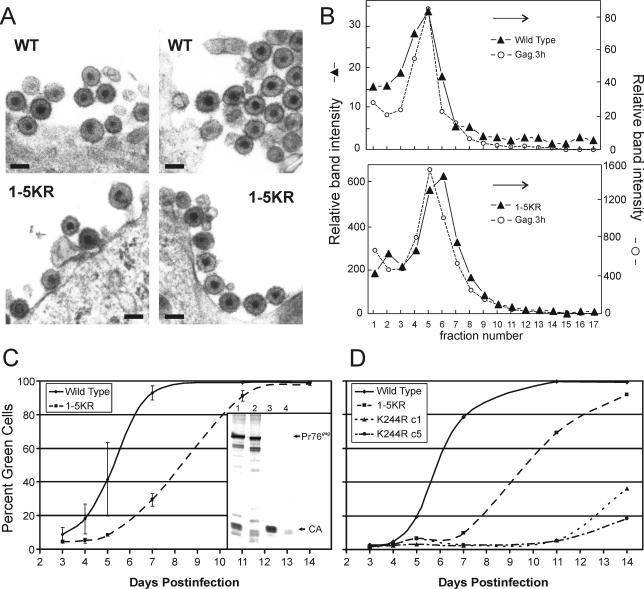
FIG. 4.
Characterization of 1-5KR virions. (A) QT6 cells infected with RC.V8 or RC.1-5KR were thin sectioned and examined by EM. 1-5KR virions are visible with a morphology similar to that of the wild type. (Magnification, ×50,000; bar, 100 nm) (B) RC.V8 and RC.1-5KR particle size was determined by sedimentation of virions through a 10 to 30% sucrose gradient for 30 min. Fractions were collected and proteins were separated by SDS-PAGE. For RC.V8 and RC.1-5KR, the amount of CA present in each fraction was determined by PhosphorImager analysis and is shown on the left y axis as arbitrary units. The amount of internal control (Gag.3h) present in each fraction was also determined by PhosphorImager analysis and is shown as arbitrary units on the right y axis. The graph is representative of three independent experiments. (C) QT6 cells were transfected with pRS.V8.eGFP or pRS.1-5KR.eGFP, and virions were collected in the medium for 24 h. Equal amounts of virions were added to DF-1 cells for 24 h. The cells were analyzed by FACS at various times postinfection to determine the percentage of green cells, indicating an infection. (Inset) An autoradiograph showing Gag proteins from cells infected for 14 days, obtained as in Fig. 2. Lanes 1 and 2 are cell lysate fractions from the wild type and 1-5KR, respectively, and lanes 3 and 4 are medium fractions from the wild type and 1-5KR, respectively. The graph shows the average of three independent experiments. (D) Lysine 244 is part of the consensus sumoylation sequence, ΨxKE, where Ψ is a hydrophobic residue and x is any residue. To destroy the potential sumoylation site, lysine 244 was changed to arginine (K244R) and cloned into RS.V8.eGFP. Two K244R clones were analyzed for infectivity as described above and were compared to the wild type and 1-5KR. A representative graph of the results of three experiments is shown.