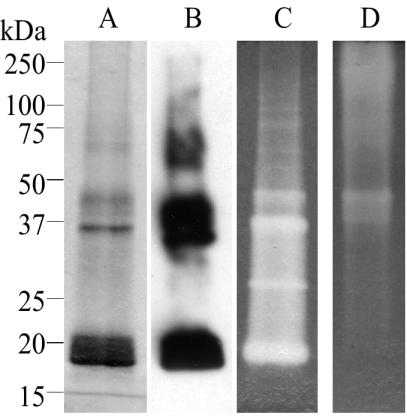
FIG. 2.
Purification and activity assay for His-SOD. His-SOD protein isolated from E. coli was electrophoresed on 10% polyacrylamide-SDS gels (A, B, and C) and a native polyacrylamide gel (D). The size standards refer to panels A, B, and C. (A) A Coomassie-stained gel of denatured purified protein. (B) Immunoblot with the His tag antibody, showing that all of the visible bands on the Coomassie-stained gel are also immunoreactive and therefore represent multiple forms of the His-SOD protein. (C and D) The activity of the recombinant protein was assayed following separation on either SDS-PAGE (panel C) or native polyacrylamide gels (panel D) in the absence of reducing agent, with the inhibition of NBT reduction technique of Beauchamp and Fridovich (10). The clear zones on the gels indicate SOD activity.