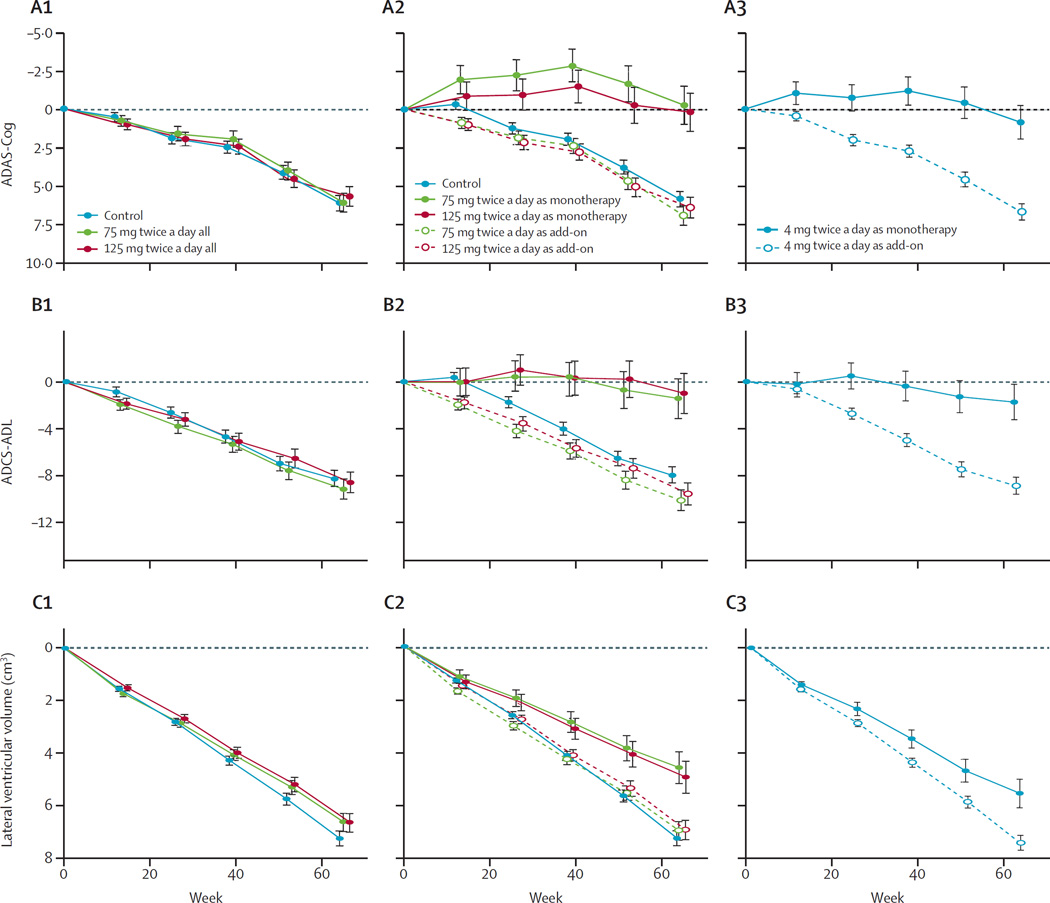
Figure 2. Least squares estimates of mean change from baseline in ADAS-Cog (A), ADCS-ADL (B), and lateral ventricular volume (C).
We did the estimates with either the primary analysis model with Alzheimer’s disease comedication status as an additive term in the model (A1, B1, C1), or a prespecified repeat of the primary analysis with Alzheimer’s disease comedication status as an interaction term in the model showing effect of leuco-methylthioninium bis(hydromethanesulfonate) treatment as either monotherapy or as add-on to existing Alzheimer’s disease treatments (A2, B2, C2). In both these analyses, the control group is as randomised. In a further non-prespecified analysis, we compared patients randomised to the control arm taking 4 mg leuco-methylthioninium bis(hydromethanesulfonate) treatment as either monotherapy or as add-on to existing Alzheimer’s disease treatments (A3, B3, C3). Numbers of participants analysed in each of the study group are shown in tables 2 and 3, and in the appendix, and numbers completing treatment with 4 mg, 75 mg, or 125 mg twice daily are shown in figure 1, according to Alzheimer’s disease comedication status. Corresponding results for ADCS-CGIC and MMSE are shown in the appendix. ADAS-Cog=Alzheimer’s Disease Assessment Scale–Cognitive Subscale. ADCS-ADL=Alzheimer’s Disease Co-operative Study–Activities of Daily Living. ADCS-CGIC=Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change scale.