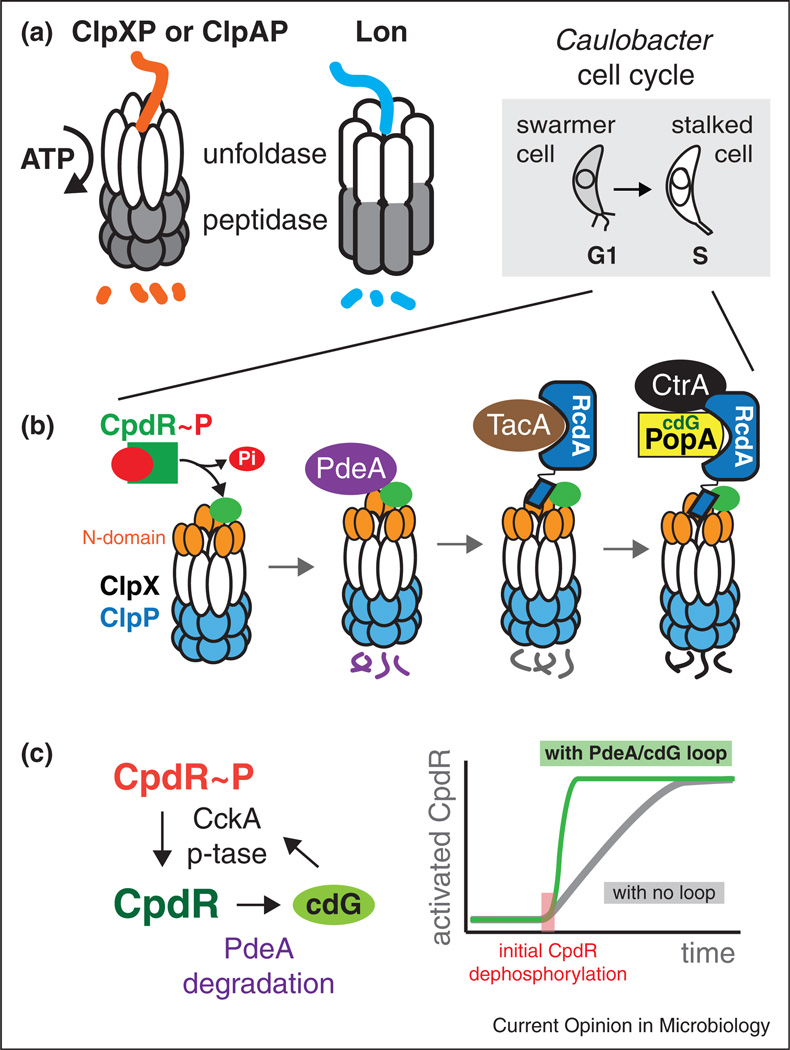
Figure 1.
Protein degradation by energy dependent proteases can be shaped by hierarchical adaptors. (a) The Clp family of proteases are composed of unfoldases (ClpX or ClpA) paired with the ClpP peptidase. The Lon protease is a single polypeptide with these activities contained in different domains. (b) The G1-S transition in Caulobacter is accompanied by morphological changes from a motile swarmer cell to a sessile stalked cell. At this transition, the dephosphorylation of CpdR initiates the assembly of an adaptor hierarchy that results in staged degradation of substrates. (c) CpdR phosphorylation (and its activity) is ultimately controlled by CckA. CckA is a histidine kinase, but high levels of cdG cause it to switch to a phosphatase, resulting in increased dephosphorylation of CpdR. CpdR is directly responsible for delivering the cdG phosphodiesterase PdeA to the ClpXP protease. This sets up a putative positive feedback loop in which initial CpdR dephosphorylation can catalyze full conversion of the CpdR pool to an activated state.