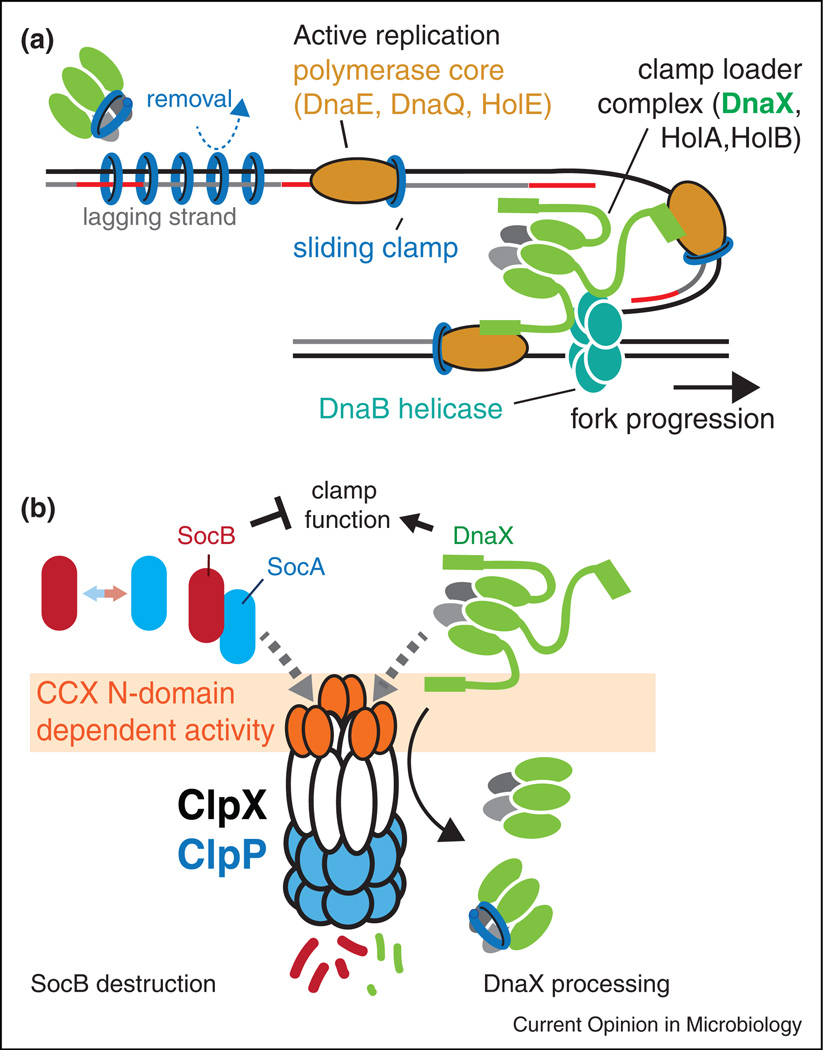
Figure 2.
Replication clamp activity is controlled in part by the ClpXP protease. (a) DnaX is the protein in clamp loader that delivers the mechanical force needed for clamp opening. The full-length clamp loader is tethered to the replication fork by interactions between the C-terminus of DnaX, DNA helicase (DnaB) and the DnaE component of the polymerase. Removal of the C-terminus would release a shortened version of the clamp loader that could act as an unloader away from the replication fork. (b) Under normal circumstances, both DnaX and SocB (with SocA acting as an adaptor) are degraded by ClpXP in an N-domain dependent manner. If the N-domain of ClpX is perturbed during damaging conditions (either by competition or direct damage) both SocB and full-length DnaX levels would rise providing compensating effects. Alternatively, if SocAB levels rise dramatically, this could itself compete for ClpXP, slowing the processing of DnaX and ensuring retention of clamp loading activity at the replication fork or damaged sites.