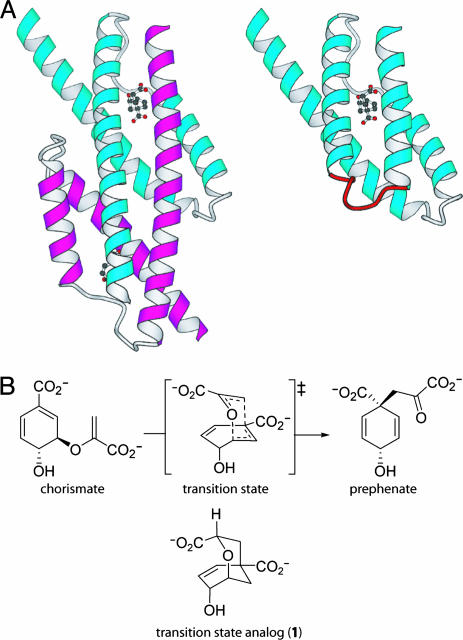
Fig. 1.
Topological redesign of the enzyme MjCM. (A) The thermostable MjCM homodimer (Left) was converted into a monomer (mMjCM, Right) by inserting a flexible hinge loop (red) into the long H1 helix (9). The models are based on the x-ray structure (20) of a related E. coli chorismate mutase domain complexed with a transition-state analog, 1 (14), which is shown in the models in ball-and-stick representation. (B) Both enzymes efficiently catalyze the rearrangement of chorismate to prephenate.