Abstract
Background
The extent of variability in treatment suggestions for melanocytic lesions made by pathologists is unknown.
Objective
We investigated how often pathologists rendered suggestions, reasons for providing suggestions, and concordance with national guidelines.
Methods
We conducted a cross-sectional survey of pathologists. Data included physician characteristics, experience, treatment recommendation practices.
Results
Of 301 pathologists, 207 (69%) from 10 states (CA, CT, HI, IA, KY, LA, NJ, NM, UT, WA), enrolled. Fifteen percent and 7% reported never and always including suggestions, respectively. Reasons for offering suggestions included improved care (79%), clarification (68%), and legal liability (39%). Reasons for not offering suggestions included referring physician preference (48%), lack of clinical information (44%), and expertise (29%). Training and caseload were associated with offering suggestions (p<0.05). Physician suggestions were most consistent for mild/moderate dysplastic nevi and melanoma. For melanoma in-situ, 18 (9%) and 32 (15%) pathologists made suggestions that under- or over-treated lesions based on NCCN guidelines, respectively. For invasive melanoma, 14 (7%) of pathologists made treatment suggestions that under-treated lesions based on NCCN guidelines.
Limitations
Treatment suggestions were self-reported.
Conclusions
Pathologists made recommendations ranging in consistency. These findings may inform efforts to reduce treatment variability and optimize patterns of care delivery for patients.
Keywords: melanoma, melanoma in situ, treatment, dermatopathology, dysplastic nevi, atypical nevi, melanocytic lesions
Introduction
Understanding why pathologists make treatment suggestions provides insight into patient care practices. Currently, little is known about how often pathologists make suggestions or whether these suggestions are consistent with national guidelines. Given wide variability in surgical and non-surgical therapies for melanocytic lesions1–3 and lack of consensus for both diagnosis and treatment of various types of “atypical/dysplastic” nevi,3–6 understanding interpreting pathologists’ suggestions could provide valuable insight. To further complicate matters, terminology for melanocytic lesions lacks standardization and is reflected in poor interobserver reproducibility for borderline tumors with unclear malignant potential.7–11 Some pathologists have also proposed to abandon the grading system of dysplastic nevi entirely, given the ambiguity of the connotations associated with “mild” or “moderate” dysplastic nevi.12 Lesions with the same diagnosis, such as Spitz nevi, can vary in pathologic characteristics and pathologists may make recommendations to better guide treatment for these lesions.
Understanding whether such motivations underlie treatment recommendations will help guide the patient’s physician in making a decision on the course of treatment. In addition, although the National Comprehensive Cancer Network (NCCN)13 has established recommendations for the treatment of melanoma in-situ and melanoma, it remains unclear whether treatments currently suggested by pathologists are in accordance with these standards.1,14–16
Recognizing variation in diagnostic thresholds, interpretation, and treatment suggestions for the wide spectrum of melanocytic skin lesions, Piepkorn et al. proposed the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis ( MPATH-Dx) classification scheme in 2014 to reduce complexity and improve pathology reporting of these neoplasms. This schema stratifies melanocytic lesions into five broad categories based on histologic findings and treatment suggestions. Example diagnoses (and suggested treatments) for the MPATH-Dx categories are as follows: dysplastic nevus with mild cytologic atypia in category 1 (no further treatment), dysplastic nevus with moderate cytologic atypia and conventional Spitz nevus in category 2 (narrow but complete re-excision suggested), dysplastic nevus with severe cytologic atypia, atypical Spitzoid lesion, and melanoma in situ in category 3 (repeat excision with at least 5mm margins suggested), and invasive melanoma in categories 4 and 5 (wide excision with at least 1cm margins).17–20
There is a knowledge gap in the reasons why and how often pathologists provide treatment suggestions. Understanding these underlying reasons and the consistency or variability in these suggestions can help a physician triage care for pigmented lesions, particularly for lesions without nationally recognized treatment guidelines. Furthermore, data on standard treatment suggestions can serve as a starting point for clinical trials aimed at understanding the appropriate treatment of pigmented lesions. The primary objective of this study is to determine how often and why practicing pathologists render treatment suggestions in their final pathology reports, what suggestions are provided, and how often the suggestions align with NCCN national guidelines for melanoma.
Methods
Data were obtained from responses to a cross-sectional survey of practicing pathologists enrolled in the M-Path study, which was designed to assess the variability in pathology diagnoses. The M-Path study survey recruitment methods have previously been described in detail.21 Briefly, a survey was sent to eligible pathologists practicing in 10 U.S. states (CA, CT, HI, IA, KY, LA, NJ, NM, UT, WA) over a one year period (July 2013-August 2014). Inclusion in the study required interpreting melanocytic skin lesions in the past year and plans to continue over the next two years. Medical students, residents, and fellows in training were ineligible.
The survey examined individual demographic and practice characteristics, including age and gender, training and type of work environment, and experience and confidence in interpreting melanocytic skin lesions. The survey also included questions on several topics relevant to the field of dermatology: whole slide digital imaging, medical malpractice, second opinions, factors influencing diagnoses, and treatment suggestions. To capture current behavior, physicians were asked how often they reported treatment suggestions regarding re-excision margins as well as reasons for inclusion/exclusion of these suggestions. To understand how treatment suggestions are being used relative to specific diagnoses, physicians were asked, “Assuming positive biopsy margins, what treatment would you recommend for the following diagnoses if the provider asked your opinion?” Respondents could then indicate their suggestions for mildly, moderately, and severely atypical dysplastic nevi, conventional Spitz nevi, atypical Spitz lesions, melanoma in situ, and invasive melanoma. A complete copy of the survey is available online at http://depts.washington.edu/epidem/Docs/Full_M-Path_BaselineSurvey.pdf.22
Responses regarding frequency of reports that include treatment suggestions were grouped a priori into four categories: 0%, 1 to 5%, 6 to 49%, and ≥50%. The Kruskal Wallis test was used to test for associations between this variable and physician characteristics. Spearman’s rank correlation was used to test for monotonic trends in the percent of total pathology reports that included treatment suggestions across physician characteristics. To investigate compliance with national guidelines for melanoma, we determined if the treatment selected by the physician was less than recommended standards (under-treatment), agreed with the national standards (congruent treatment), or represented surgical margins beyond those suggested by national standards (over-treatment). Treatment suggestions for melanoma in situ were treated as an ordinal variable (under treatment, agree, over treatment) and analyzed using the Kruskall Wallis test to determine associations with physician characteristics. Associations between invasive melanoma treatment suggestions and physician characteristics were assessed using Chi-square or Fisher’s exact test where appropriate. All analyses were conducted using SAS version 9.4 (Cary, NC), and all statistical tests were 2 tailed with α = .05.
Results
Of 301 eligible pathologists, 207 (69%) enrolled in the study. The mean age of participants was 51 years, with 123 men (59%) and 59 individuals (29%) affiliated with an academic medical center. Board certification or fellowship training in dermatopathology was reported by 81 (39%) (Table 1). Of 207 survey respondents, 79 (38%) indicated that they were board certified in dermatopathology. Of these 79, 21 (27%) also indicated that they were board certified in dermatology. Of the 21, 4 (19%) noted that they also were board certified in anatomic and clinical pathology. Thus, 17 of the 207 survey respondents (8%) completed dermatology residencies while 4 of the 207 survey respondents (2%) completed both dermatology and pathology residencies.
Table 1.
Characteristics of M-Path study pathologists by treatment suggestion practices
| Number of pathologists (Percent) Survey response describing the percent of their final pathology reports that include treatment suggestions |
|||||
|---|---|---|---|---|---|
| Physician Characteristics | Total | 0 | 1–5 | 6–49 | ≥50 |
| Demographics | 207 | 32 (15%) | 50 (24%) | 73 (35%) | 52 (25%) |
| Age (yrs.) | |||||
| <50 | 95 | 16 (17%) | 19 (20%) | 39 (41%) | 21 (22%) |
| ≥50 | 112 | 16 (14%) | 31 (28%) | 34 (30%) | 31 (28%) |
| Gender | |||||
| Female | 84 | 12 (14%) | 20 (24%) | 28 (33%) | 24 (29%) |
| Male | 123 | 20 (16%) | 30 (24%) | 45 (37%) | 28 (23%) |
| Training and Experience | |||||
| Affiliation with academic medical center | |||||
| No | 148 | 25 (17%) | 39 (26%) | 49 (33%) | 35 (24%) |
| Yes | 59 | 7 (12%) | 11 (19%) | 24 (41%) | 17 (29%) |
| Training* | |||||
| Board certified or fellowship trained in Dermatopathology*** | 81 | 3 (4%) | 22 (27%) | 37 (46%) | 19 (23%) |
| Other board certification or fellowship training **** | 126 | 29 (23%) | 28 (22%) | 36 (29%) | 33 (26%) |
| Number years interpreting MSL lesions | |||||
| <10 | 80 | 14 (18%) | 15 (19%) | 34 (43%) | 17 (21%) |
| 10–19 | 63 | 6 (10%) | 21 (33%) | 20 (32%) | 16 (25%) |
| ≥20 | 64 | 12 (19%) | 14 (22%) | 19 (30%) | 19 (30%) |
| Percent of caseload interpreting melanocytic skin lesions* ** | |||||
| <10% | 90 | 20 (22%) | 25 (28%) | 24 (27%) | 21 (23%) |
| ≥10% | 117 | 12 (10%) | 25 (21%) | 49 (42%) | 31 (26%) |
| Average number of cases of melanoma interpreted per month | |||||
| <5 | 91 | 14 (15%) | 24 (26%) | 24 (26%) | 29 (32%) |
| ≥5 | 116 | 18 (16%) | 26 (22%) | 49 (42%) | 23 (20%) |
| Average number of benign melanocytic skin lesions interpreted per month * | |||||
| <50 | 99 | 21 (21%) | 22 (22%) | 29 (29%) | 27 (27%) |
| ≥50 | 108 | 11 (10%) | 28 (26%) | 44 (41%) | 25 (23%) |
| Number of second opinions requested by physician per month | |||||
| <5 | 117 | 18 (15%) | 30 (26%) | 34 (29%) | 35 (30%) |
| ≥5 | 90 | 14 (16%) | 20 (22%) | 39 (43%) | 17 (19%) |
| In general, how confident are you in assessments of melanocytic skin lesions? | |||||
| Confident | 178 | 25 (14%) | 47 (26%) | 63 (35%) | 43 (24%) |
| Not confident | 29 | 7 (24%) | 3 (10%) | 10 (34%) | 9 (31%) |
P-values for Kruskal-Wallis test were ≤ 0.05.
P-value for Spearman rank correlation test for trend was ≤ 0.05.
This category consists of physicians with single or multiple fellowships that include dermatopathology. Also includes physicians with single or multiple board certifications that include dermatopathology.
Other includes fellowships or board certifications in surgical pathology, cytopathology, hematopathology, etc.
Pathologists varied in their inclusion of treatment suggestions in final pathology reports. Approximately 15% (n=32) reported never including treatment suggestions, while the remainder included treatment suggestions by varying degrees: 24% of pathologists (n=50) reported including suggestions in 1–5% of reports, 35% (n=73) reported including suggestions in 6–49% of reports, and 25% (n=52) reported including suggestions ≥ 50% of reports. Among the 52 pathologists who included suggestions in the majority of their reports, 14 (7%) reported always including them. Reasons given for providing suggestions included (1) improved patient care (n=164, 79%), (2) clarification of treatment options for the biopsying clinician (n=140, 68%), and (3) protection from legal liability (n=81, 39%). Reasons for not providing suggestions in reports included (1) referring physicians did not want recommendations in the pathology report (n=100, 48%), (2) insufficient clinical information (n=92, 44%), and (3) lack of requisite clinical expertise (n=60, 29%). Pathologists significantly more likely to include treatment suggestions were board certified or fellowship trained in dermatopathology as well as those with higher melanocytic lesion caseload (≥10%). For mild and moderate dysplastic nevi, pathologists with board certification or considered an expert by their colleagues trended towards offering more conservative treatment recommendations (both p<0.05).
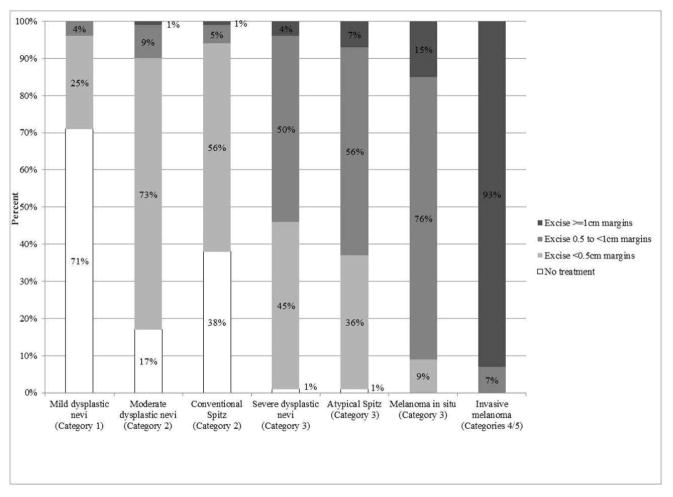
The distribution of suggested treatments by melanocytic lesion type is shown in Figure 1. Pathologists most consistently agreed with MPATH-Dx treatment suggestions when considering diagnoses in the lowest (mildly dysplastic nevus) and highest (invasive melanoma) categories of the MPATH-Dx spectrum. Seventy-one percent of physicians recommended no further treatment for the MPATH-Dx Category 1 dysplastic nevi with mild cytologic atypia, consistent with MPATH-Dx suggestions and 73% of physicians recommended excising a MPATH-Dx Category 2 dysplastic nevi with moderate cytologic atypia lesion with margins <0.5cm. For invasive melanoma, 93% of physicians agreed with suggestions for treating with margins ≥1cm. For melanoma in situ, 76% recommended excision with 0.5 to 1cm margins, consistent with the MPATH-Dx suggestion and NCCN guidelines. Lesions with more variation in the recommended treatment were Spitz nevi, dysplastic nevi with severe cytologic atypia, and atypical Spitz tumors, where each had <60% agreement with the MPATH-Dx recommendations.
Figure 1.
Treatment suggestions of practicing U.S. pathologists based on type of melanocytic lesion (categories 1 through 5 refer to categories of the MPATH-Dx schema).
For melanoma in situ, 18 (9%) and 32 (15%) pathologists made treatment suggestions that under- or over-treated lesions based on NCCN guidelines, respectively (Table 2). Pathologists with board certification or fellowship training in dermatopathology and a monthly caseload of ≥50% benign melanocytic skin lesions were more likely to suggest treatments consistent with the NCCN guidelines for melanoma in situ treatment (p<0.001, =0.001 respectively). Those who undertreated MIS tended to have fewer years of experience interpreting melanocytic lesions, read <50 benign melanocytic lesions per month, and had a caseload of <10% melanocytic lesions per month (Table 2).
Table 2.
Pathologist treatment suggestions compared to NCCN**** guidelines for melanoma in situ or invasive melanoma by pathologist characteristics
| Pathologist characteristics | Melanoma in situ | Invasive melanoma | |||
|---|---|---|---|---|---|
| Under-treatment | Agree with NCCN treatment guidelines | Over-treatment | Under-treatment | Agree with NCCN treatment guidelines | |
| Total | 18 (9%) | 157 (76%) | 32 (15%) | 14 (7%) | 193 (93%) |
| Age (yrs.) | |||||
| <50 | 7 (7%) | 72 (76%) | 16 (17%) | 2 (2%)* | 93 (98%)* |
| ≥50 | 11 (10%) | 85 (76%) | 16 (14%) | 12 (11%)* | 100 (89%)* |
| Gender | |||||
| Female | 5 (6%) | 61 (73%) | 18 (21%) | 4 (5%) | 80 (95%) |
| Male | 13 (11%) | 96 (78%) | 14 (11%) | 10 (8%) | 113 (92%) |
| Training and experience Affiliation with academic medical center | |||||
| No | 13 (9%) | 112 (76%) | 23 (16%) | 12 (8%) | 136 (92%) |
| Yes | 5 (8%) | 45 (76%) | 9 (15%) | 2 (3%) | 57 (97%) |
| Training | |||||
| Board certified or fellowship trained in dermatopathology ** | 4 (5%)* | 73 (90%)* | 4 (5%)* | 3 (4%) | 78 (96%) |
| Other board certification or fellowship training *** | 14 (11%)* | 84 (67%)* | 28 (22%)* | 11 (9%) | 115 (91%) |
| Number years interpreting melanocytic skin lesions | |||||
| <10 | 7 (9%) | 58 (73%) | 15 (19%) | 1 (1%)* | 79 (99%)* |
| 10–19 | 3 (5%) | 52 (83%) | 8 (13%) | 5 (8%)* | 58 (92%)* |
| ≥20 | 8 (13%) | 47 (73%) | 9 (14%) | 8 (13%)* | 56 (88%)* |
| Percent of caseload interpreting melanocytic skin lesions | |||||
| <10% | 9 (10%) | 64 (71%) | 17 (19%) | 9 (10%) | 81 (90%) |
| ≥10% | 9 (8%) | 93 (79%) | 15 (13%) | 5 (4%) | 112 (96%) |
| Average number of benign melanocytic skin lesions interpreted per month | |||||
| <50 | 12 (12%)* | 64 (65%)* | 23 (23%)* | 8 (8%) | 91 (92%) |
| ≥50 | 6 (6%)* | 93 (86%)* | 9 (8%)* | 6 (6%) | 102 (94%) |
| Number of second opinions requested by physician per month | |||||
| <5 | 9 (8%) | 93 (79%) | 15 (13%) | 7 (6%) | 110 (94%) |
| ≥5 | 9 (10%) | 64 (71%) | 17 (19%) | 7 (8%) | 83 (92%) |
| In general, how confident are you in assessments of melanocytic skin lesions? | |||||
| Confident | 17 (10%) | 135 (76%) | 26 (15%) | 12 (7%) | 166 (93%) |
| Not confident | 1 (3%) | 22 (76%) | 6 (21%) | 2 (7%) | 27 (93%) |
P-values for Kruskal-Wallis, Chi-square of Fishers exact where appropriate were ≤ 0.05.
This category consists of physicians with single or multiple fellowships that include dermatopathology. Also includes physicians with single or multiple board certifications that include dermatopathology.
Other includes fellowships or board certifications in surgical pathology, cytopathology, hematopathology, etc.
American Joint Committee on Cancer / National Comprehensive Cancer Network.
For invasive melanoma, 14 (7%) of pathologists made treatment suggestions that would have under-treated lesions based on NCCN guidelines. Pathologist characteristics associated with treatment suggestions inconsistent with NCCN guidelines for invasive melanoma were age ≥50 years (p=0.02) and more years of experience interpreting melanocytic skin lesions (p=0.03; Table 2).
Discussion
Our results show variability in providing treatment suggestions among pathologists. In addition, this study highlights the wide range of treatment suggestions for melanocytic lesions that lack national treatment guidelines.23,24 Physician experience and training influenced whether pathologists made suggestions, with dermatopathology training trending towards more conservative treatment suggestions for mild and moderately dysplastic nevi. For melanomas and melanoma in situ, pathologists typically made treatment suggestions in accordance with the established NCCN guidelines. 25,26 However, some pathologists made recommendations that would have under- or over-treated these melanocytic lesions.
A group of pathologists (15%) replied that they never made treatment suggestions, while another group of pathologists (7%) always did. Patient care motivations, including improving patient care and clarifying treatment suggestions, represented prominent reasons for making recommendations.27 Preference of the referring physician was the main reason for not making treatment suggestions, with 48% of pathologists noting this as the primary reason. For pathologists who do not make treatment suggestions, other reasons included not having enough clinical information or expertise. Pathologists with more clinical experience (e.g., dermatopathology training and higher monthly caseload of melanocytic lesions) were more likely to include treatment suggestions in their report. We also noted that older pathologists and those with more years interpreting these lesions were less likely to follow the NCCN treatment guidelines. Experience may reflect a pathologist’s comfort level with making the diagnosis in borderline lesions, or their ability to detect histologic features that could influence treatment plans. Considerations such as malpractice liability may also play a role, as discussed elsewhere.28
The lack of diagnostic and treatment consensus for some types of dysplastic nevi and Spitz lesions is reflected in this survey. 1–3,17,29–45 Pathologists may prefer to call borderline lesions “atypical” instead of “malignant”, while also erring on the side of caution and recommending re-excision with appropriate margins. This strategy ensures that the tumor is treated with surgical excision while also preventing the patient from carrying a malignant cancer diagnosis in their medical record.
Of most concern is the lack of consistency in treatment suggestions for melanoma in situ and invasive melanoma, with 24% of pathologists’ recommendations differing from NCCN criteria for melanoma in situ. Although the majority (90%) of board certified or fellowship trained pathologists suggested treatment in agreement with guidelines for melanoma in situ, only 67% of physicians with board certification or training in a different specialty matched the treatment recommendations. Another potential explanation for inconsistencies in recommendations and national guidelines may be changes in the treatment of melanoma over time, as evidenced by the Surveillance, Epidemiology, and End Results (SEER) data.46–48 From 1973–1985, the majority of melanoma in situ cases were treated with excisional biopsy. In contrast, from 1996–2006, the majority of melanoma in situ cases were treated with <1cm excisional margins, which is consistent with NCCN treatment guidelines.48 In our study, 9% of pathologists recommended under-treatment of melanoma in situ.
This study has several limitations. We assessed self-reported perceptions, not actual clinical practice; thus, some response bias could affect these findings. In addition, our findings cannot be generalized to other pathology subspecialties. The survey asked for treatment suggestions, assuming the provider was to ask the pathologist for a recommendation. Another limitation is the considerable variability within lesions of the same category such as atypical Spitz tumors.8 Pathologists examine slides and use the degree of atypia in formulating their treatment suggestions. Our survey did not provide photographs or slides of actual lesions to help pathologists guide treatment suggestions. Treatment recommendations are also frequently more consistent than diagnostic terminology. Our study provided a uniform diagnostic terminology, and a list of treatment recommendation options. Treatment recommendations may be more consistent when pathologists are examining actual slides as compared with our study.18,19 Further, the treatment options on the MPATH survey are not always reflective of everyday practice for pathologists.19 Pathologists were limited to choosing from limited options for treatment recommendations. Thus, a pathologist who normally recommends “re-excision with appropriate margins” without giving specific margins may have checked “no recommendation” based on the treatment options presented by the survey.
Strengths of our study included a survey response rate of 69% of eligible invitees, which is higher than national standards for physician surveys.49 Our data were also gathered from ten diverse geographic U.S. states and included responses from both academic and community pathologists.
Our study results may largely reflect uncertainty in the evidence-based literature regarding treatment of these lesions. Our findings point to a potential gap in use of guideline treatment recommendations and also the persistent challenge that clinicians face with the intermediate melanocytic lesions.
Acknowledgments
Funding Source: This work was supported by the National Cancer Institute (R01 CA151306, KO5 CA104699). The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institutes of Health.
This study was approved by the Institutional Review Boards of the University of Washington, Fred Hutchinson Cancer Research Center, Oregon Health & Sciences University, Rhode Island Hospital, and Dartmouth College.
Footnotes
Conflict of Interest Disclosure: Dr. Lott is an employee of Bayer HealthCare Pharmaceuticals, which had no involvement in this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30:513–20. doi: 10.1016/s0046-8177(99)90193-4. [DOI] [PubMed] [Google Scholar]
- 2.Su LD, Fullen DR, Sondak VK, Johnson TM, Lowe L. Sentinel lymph node biopsy for patients with problematic spitzoid melanocytic lesions: a report on 18 patients. Cancer. 2003;97:499–507. doi: 10.1002/cncr.11074. [DOI] [PubMed] [Google Scholar]
- 3.Chen S. The dysplastic nevus controversy: It is not about the nevus per se but one’s belief in the multistep tumorigenesis theory. Am J Dermatopathol. 2010;32:858. doi: 10.1097/DAD.0b013e3181dc0fda. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus conference. Diagnosis and treatment of early melanoma. JAMA. 1992;268:1314–9. doi: 10.1001/jama.1992.03490100112037. [DOI] [PubMed] [Google Scholar]
- 5.Diagnosis and treatment of early melanoma. NIH Consensus Development Conference January 27–29, 1992 Consensus statement / NIH Consensus Development Conference National Institutes of Health Consensus Development Conference; 1992; pp. 1–25. [PubMed] [Google Scholar]
- 6.Cook MG, Clarke TJ, Humphreys S, et al. The evaluation of diagnostic and prognostic criteria and the terminology of thin cutaneous malignant melanoma by the CRC Melanoma Pathology Panel. Histopathology. 1996;28:497–512. doi: 10.1046/j.1365-2559.1996.d01-464.x. [DOI] [PubMed] [Google Scholar]
- 7.Nobre AB, Pineiro-Maceira J, Luiz RR. Analysis of interobserver reproducibility in grading histological patterns of dysplastic nevi. Anais brasileiros de dermatologia. 2013;88:23–31. doi: 10.1590/S0365-05962013000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patrawala S, Maley A, Greskovich C, et al. Discordance of histopathologic parameters in cutaneous melanoma: Clinical implications. J Am Acad Dermatol. 2016;74:75–80. doi: 10.1016/j.jaad.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Zembowicz A, Scolyer RA. Nevus/Melanocytoma/Melanoma: an emerging paradigm for classification of melanocytic neoplasms? Arch Pathol Lab Med. 2011;135:300–6. doi: 10.5858/2010-0146-RA.1. [DOI] [PubMed] [Google Scholar]
- 10.Ludgate MW, Fullen DR, Lee J, et al. The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer. 2009;115:631–41. doi: 10.1002/cncr.24047. [DOI] [PubMed] [Google Scholar]
- 11.Mones JM, Ackerman AB. “Atypical” Spitz’s nevus, “malignant” Spitz’s nevus, and “metastasizing” Spitz’s nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:310–33. doi: 10.1097/00000372-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Lozeau DF, Farber MJ, Lee JB. A nongrading histologic approach to Clark (dysplastic) nevi: A potential to decrease the excision rate. J Am Acad Dermatol. 2016;74:68–74. doi: 10.1016/j.jaad.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 13.American Cancer S, National Comprehensive Cancer N. Melanoma: treatment guidelines for patients (Part 1) Dermatology nursing / Dermatology Nurses’ Association. 2005;17:119–31. [PubMed] [Google Scholar]
- 14.Barnhill RL, Cerroni L, Cook M, et al. State of the art, nomenclature, and points of consensus and controversy concerning benign melanocytic lesions: outcome of an international workshop. Adv Anat Pathol. 2010;17:73–90. doi: 10.1097/PAP.0b013e3181cfe758. [DOI] [PubMed] [Google Scholar]
- 15.Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol. 1996;27:528–31. doi: 10.1016/s0046-8177(96)90157-4. [DOI] [PubMed] [Google Scholar]
- 16.Piepkorn M. On the nature of histologic observations: the case of the Spitz nevus. J Am Acad Dermatol. 1995;32:248–54. doi: 10.1016/0190-9622(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 17.Piepkorn MW, Barnhill RL, Elder DE, et al. The MPATH-Dx reporting schema for melanocytic proliferations and melanoma. J Am Acad Dermatol. 2014;70:131–41. doi: 10.1016/j.jaad.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lott JP, Elmore JG, Zhao GA, et al. Evaluation of the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis (MPATH-Dx) classification scheme for diagnosis of cutaneous melanocytic neoplasms: Results from the International Melanoma Pathology Study Group. J Am Acad Dermatol. 2016 doi: 10.1016/j.jaad.2016.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elston DM. Management of atypical pigmented lesions. J Am Acad Dermatol. 2014;70:142–5. doi: 10.1016/j.jaad.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Stigall LE, Zitelli JA. Surgical margins for possibly malignant melanocytic lesions. J Am Acad Dermatol. 2014;71:588–9. doi: 10.1016/j.jaad.2014.04.073. [DOI] [PubMed] [Google Scholar]
- 21.Onega T, Reisch LM, Frederick PD, et al. Use of Digital Whole Slide Imaging in Dermatopathology. J Digit Imaging. 2016;29:243–53. doi: 10.1007/s10278-015-9836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore Joann. University of Washington; 2016. [Accessed Mar 4, 2016]. at http://depts.washington.edu/epidem/Docs/Full_M-Path_BaselineSurvey.pdf. [Google Scholar]
- 23.Kim CC, Swetter SM, Curiel-Lewandrowski C, et al. Addressing the knowledge gap in clinical recommendations for management and complete excision of clinically atypical nevi/dysplastic nevi: Pigmented Lesion Subcommittee consensus statement. JAMA dermatology. 2015;151:212–8. doi: 10.1001/jamadermatol.2014.2694. [DOI] [PubMed] [Google Scholar]
- 24.Strazzula L, Vedak P, Hoang MP, Sober A, Tsao H, Kroshinsky D. The utility of re-excising mildly and moderately dysplastic nevi: a retrospective analysis. J Am Acad Dermatol. 2014;71:1071–6. doi: 10.1016/j.jaad.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2. 2013: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11:395–407. doi: 10.6004/jnccn.2013.0055. [DOI] [PubMed] [Google Scholar]
- 26.Houghton A, Coit D, Bloomer W, et al. NCCN melanoma practice guidelines. National Comprehensive Cancer Network Oncology. 1998;12:153–77. [PubMed] [Google Scholar]
- 27.Orfanos CE. From Hippocrates to modern medicine. Journal of the European Academy of Dermatology and Venereology : JEADV. 2007;21:852–8. doi: 10.1111/j.1468-3083.2007.02273.x. [DOI] [PubMed] [Google Scholar]
- 28.Carney PA, Frederick PD, Reisch LM, et al. How concerns and experiences with medical malpractice affect dermatopathologists’ perceptions of their diagnostic practices when interpreting cutaneous melanocytic lesions. Journal of the American Academy of Dermatology. 2016;74:317–24. doi: 10.1016/j.jaad.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemente C, Cochran AJ, Elder DE, et al. Histopathologic diagnosis of dysplastic nevi: concordance among pathologists convened by the World Health Organization Melanoma Programme. Hum Pathol. 1991;22:313–9. doi: 10.1016/0046-8177(91)90078-4. [DOI] [PubMed] [Google Scholar]
- 30.Meyer LJ, Piepkorn M, Goldgar DE, et al. Interobserver concordance in discriminating clinical atypia of melanocytic nevi, and correlations with histologic atypia. J Am Acad Dermatol. 1996;34:618–25. doi: 10.1016/s0190-9622(96)80061-2. [DOI] [PubMed] [Google Scholar]
- 31.Piepkorn MW, Barnhill RL, Cannon-Albright LA, et al. A multiobserver, population-based analysis of histologic dysplasia in melanocytic nevi. J Am Acad Dermatol. 1994;30:707–14. doi: 10.1016/s0190-9622(08)81499-5. [DOI] [PubMed] [Google Scholar]
- 32.Duncan LM, Berwick M, Bruijn JA, Byers HR, Mihm MC, Barnhill RL. Histopathologic recognition and grading of dysplastic melanocytic nevi: an interobserver agreement study. The Journal of investigative dermatology. 1993;100:318S–21S. doi: 10.1111/1523-1747.ep12470215. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes AR, Mihm MC, Jr, Weinstock MA. Dysplastic melanocytic nevi: a reproducible histologic definition emphasizing cellular morphology. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1989;2:306–19. [PubMed] [Google Scholar]
- 34.Weinstock MA, Barnhill RL, Rhodes AR, Brodsky GL. Reliability of the histopathologic diagnosis of melanocytic dysplasia. The Dysplastic Nevus Panel Arch Dermatol. 1997;133:953–8. [PubMed] [Google Scholar]
- 35.Zedek DC, McCalmont TH. Spitz nevi, atypical spitzoid neoplasms, and spitzoid melanoma. Clinics in laboratory medicine. 2011;31:311–20. doi: 10.1016/j.cll.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Barnhill RL. The Spitzoid lesion: rethinking Spitz tumors, atypical variants, ‘Spitzoid melanoma’ and risk assessment. Mod Pathol. 2006;19(Suppl 2):S21–33. doi: 10.1038/modpathol.3800519. [DOI] [PubMed] [Google Scholar]
- 37.Murphy ME, Boyer JD, Stashower ME, Zitelli JA. The surgical management of Spitz nevi. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2002;28:1065–9. doi: 10.1046/j.1524-4725.2002.02067.x. discussion 9. [DOI] [PubMed] [Google Scholar]
- 38.Murali R, Sharma RN, Thompson JF, et al. Sentinel lymph node biopsy in histologically ambiguous melanocytic tumors with spitzoid features (so-called atypical spitzoid tumors) Ann Surg Oncol. 2008;15:302–9. doi: 10.1245/s10434-007-9577-3. [DOI] [PubMed] [Google Scholar]
- 39.Miteva M, Lazova R. Spitz nevus and atypical spitzoid neoplasm. Semin Cutan Med Surg. 2010;29:165–73. doi: 10.1016/j.sder.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Lohmann CM, Coit DG, Brady MS, Berwick M, Busam KJ. Sentinel lymph node biopsy in patients with diagnostically controversial spitzoid melanocytic tumors. The American journal of surgical pathology. 2002;26:47–55. doi: 10.1097/00000478-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Busam KJ, Murali R, Pulitzer M, et al. Atypical spitzoid melanocytic tumors with positive sentinel lymph nodes in children and teenagers, and comparison with histologically unambiguous and lethal melanomas. The American journal of surgical pathology. 2009;33:1386–95. doi: 10.1097/PAS.0b013e3181ac1927. [DOI] [PubMed] [Google Scholar]
- 42.Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions part II. Natural history and management. J Am Acad Dermatol. 2011;65:1087–92. doi: 10.1016/j.jaad.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lallas A, Kyrgidis A, Ferrara G, et al. Atypical Spitz tumours and sentinel lymph node biopsy: a systematic review. Lancet Oncol. 2014;15:e178–83. doi: 10.1016/S1470-2045(13)70608-9. [DOI] [PubMed] [Google Scholar]
- 44.Abraham RM, Karakousis G, Acs G, et al. Lymphatic invasion predicts aggressive behavior in melanocytic tumors of uncertain malignant potential (MELTUMP) The American journal of surgical pathology. 2013;37:669–75. doi: 10.1097/PAS.0b013e318288ff47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerroni L, Barnhill R, Elder D, et al. Melanocytic tumors of uncertain malignant potential: results of a tutorial held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. The American journal of surgical pathology. 2010;34:314–26. doi: 10.1097/PAS.0b013e3181cf7fa0. [DOI] [PubMed] [Google Scholar]
- 46.Higgins HW, 2nd, Lee KC, Galan A, Leffell DJ. Melanoma in situ: Part II. Histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193–203. doi: 10.1016/j.jaad.2015.03.057. quiz -4. [DOI] [PubMed] [Google Scholar]
- 47.Higgins HW, 2nd, Lee KC, Galan A, Leffell DJ. Melanoma in situ: Part I. Epidemiology, screening, and clinical features. J Am Acad Dermatol. 2015;73:181–90. doi: 10.1016/j.jaad.2015.04.014. quiz 91–2. [DOI] [PubMed] [Google Scholar]
- 48.Mocellin S, Nitti D. Cutaneous melanoma in situ: translational evidence from a large population-based study. The oncologist. 2011;16:896–903. doi: 10.1634/theoncologist.2010-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–36. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]