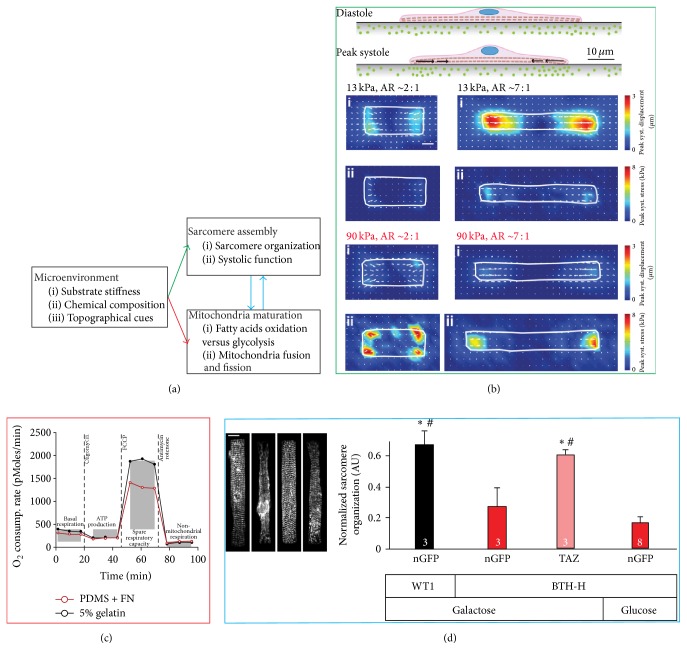
Figure 1.
Mechanosensitive control of cardiomyocytes metabolism. (a) Putative link between microenvironmental cues, sarcomere assembly, and mitochondria maturation. (b) Traction force microscopy experiments suggesting that in response to stiffening of their microenvironment cardiomyocytes may regulate their shape to maintain an optimal work output. (c) Metabolic flux analysis showing the cardiomyocytes cultured on soft substrates stiffness (5% gelatin, black) retained a greater respiratory capacity than on stiffer ones (polydimethylsiloxane, PDMS, red). (d) Qualitative and quantitative analysis of cardiomyocytes structure in a disease-on-a-chip study of Barth syndrome, an inherited cardiomyopathy caused by a mutation to the tafazzin gene. The analysis showed that hiPS-derived cardiomyocytes obtained from a Barth syndrome patient (BTH-H, nGFP) had significantly impaired contractile architecture with respect to cardiomyocytes obtained from a healthy individual (WT1, nGFP). Furthermore, introducing wild-type tafazzin (BTH-H, TAZ), but not restoring the basal ATP level by switching to the glycolytic pathway (BTH-H, Glucose), in diseased cells rescued the structural phenotype. Scale bar 10 μm. Together, these findings support the notion that mechanotransductive processes like sarcomerogenesis and myofibrillogenesis are linked not only to metabolism but also to mitochondria structure and function. Images were obtained with permission from the following sources: (b) McCain et al., 2014a [17]; (c) McCain et al., 2014b [20]; and (d) Wang et al., 2014 [21]. ∗,#Significant (p < 0.05) differences with respect to the nGFP-galactose and nGFP-glucose groups, respectively.