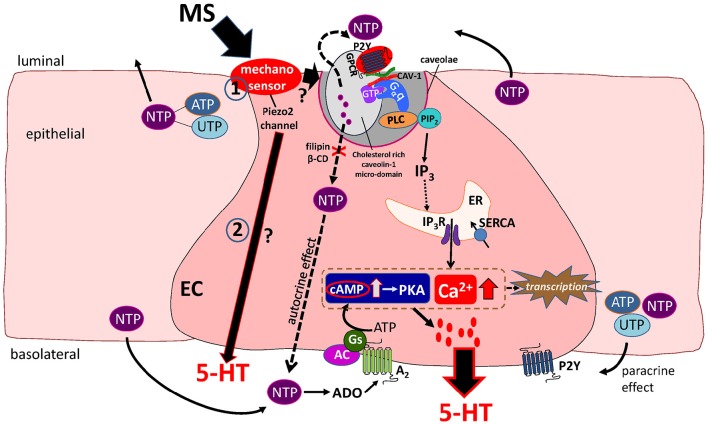
Figure 1.
Working Hypothesis of mechanotransduction in EC cells. Mechanical stress (MS) activates a mechanosensor in EC and epithelial cells to induce release of 5′nucleotide triphosphates (NTP) such as ATP and UTP that act in an autocrine or paracrine manner to modulate 5-HT release. The Piezo 2 mechanogated channel was recently identified as a critical component of the mechanosensor and mechanotransduction signaling pathway activated by MS in EC cells leading to 5-HT release (in pathways 1 and 2 in the diagram). Mechanically evoked NTP release activates a predominant P2Y1/Gαq/PLC/PIP2/IP3/IP3R/SERCA pump–Ca2+signaling pathway leading to 5-HT release. Caveolin-1 (CAV-1) associated with cholesterol-rich micro-domains in caveolae (specialized invaginations in the lipid bilayer of the EC cell membrane) forms a scaffold to support the functional coupling of the P2Y1-GPCR, Gαq, PLC and NTP secretion from the cell. In this model, caveolae and cholesterol rich caveolin-1 microdomains are essential for both NTP release and down-stream Ca2+dependent 5-HT release. Therefore, manipulations that disrupt the structure or assembly of caveolae by treating cells to filipin or β-cyclodextrin (β-CD) prevent the mechanically evoked NTP (ATP) and 5-HT secretion. A minor mechanosensitive pathway is an A2/Gs/AC-cAMP/PKA signaling pathway of 5-HT release. Ca2+ and cAMP-dependent transcriptional regulation occurs in response to mechanical stimulation that can further modulate EC cell function(s). In this model, it is postulated that Piezo 2 activation could also stimulate 5-HT secretion via a separate purine-independent pathway (pathway 2 in diagram).