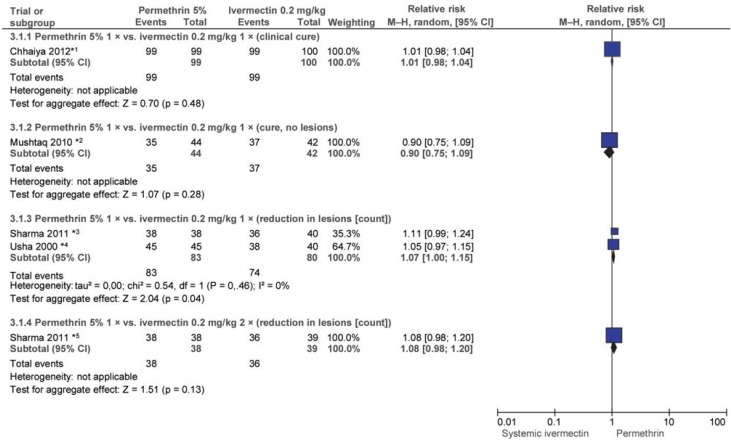
eFigure 3.
eFigure 3: Efficacy of single-dose permethrin 5% (PER) vs. 1 or 2 doses of ivermectin (IVER) 0.2 mg/kg after 4 weeks
*1 Patients not successfully cured underwent repeat treatment (weeks 1 to 4; n/N not reported).
*2 Patients not successfully cured underwent repeat treatment (week 2; n/N not reported).
*3 All patients were treated every 2 weeks.
*4 One patient in the PER group and 12 patients in the IVER group underwent repeat treatment after 2 weeks.
*5 All patients were treated every 2 weeks.
Adverse events (AEs) were reported in 5 of the 6 trials: in 2 trials (e38, e40) there were no AEs; in 2 trials one and 3 patients respectively reported a burning sensation (PER), and one and 4 respectively reported headache and pruritus (one patient) and dizziness (2 patients; systemic IVER) (e39, e42). In one other trial, headache, pruritus, and bacterial infections were reported in 7 patients (IVER), and erythema in one patient (PER) (e41).
Ivermectin 0.2 mg/kg single-dose versus 2 doses
In the 3-arm trial by Sharma and Singal (e43), patients in the third arm received 2 doses of ivermectin 0.2 mg/kg. No statistically significant difference in efficacy was found between this and a single dose after 4 weeks. Efficacy was measured using the outcome parameter “=50% improvement in lesion count” (RR: 0.97; 95% CI: [0.85; 1.12]).
Permethrin 5% versus ivermectin 1% versus IVER 0.2 mg/kg
Chhaiya et al. (e39) investigated ivermectin 1% topical versus permethrin 5% topical and ivermectin systemic (all single dose). After 4 weeks all patients were cured and there was no statistically significant difference in favor of either permethrin or systemic ivermectin (IVER 1% versus PER 5%: RR: 0.99; 95% CI: [0.96; 1.02]); IVER 1% versus IVER 0.2 mg/kg: RR: 1.01; 95% CI: [0.98; 1.04]). Patients whose treatment was unsuccessful underwent repeat treatment in weeks 1, 2, 3, and 4 (number not reported).
Ivermectin 0.15 to 0.2 mg/kg versus benzyl benzoate (BB) 10%/12.5%/25%
Five trials conducted in Nigeria, Senegal, and Oceania evaluated the efficacy of ivermectin versus BB at various doses and frequencies of administration. Some outcome parameters varied between trials (efigure 4).
Ly et al. (e44) compared one and two doses of BB 12.5% with IVER 0.5 to 0.2 mg/kg. After one week all patients whose condition had worsened substantially underwent one further treatment. After 2 and 4 weeks BB was found to be superior (efigure 4). In the BB groups 18% and 37% of patients respectively reported skin irritation during treatment. Nnoruka and Agu (e45) compared IVER 0.2 mg/kg to BB 25%, both single-dose. After 2 and 4 weeks ivermectin was found to be superior (efigure 4). Seven patients in the BB group reported irritation and pruritus (e45). It was reported that there were no AEs in the IVER group (e45).
Brooks and Grace (e46) also compared single-dose BB 10% to single-dose IVER 0.2 mg/kg; this trial included only children. There was no statistically significant difference after 3 weeks (efigure 4). Considerably more cases of skin irritation were reported in the BB group.
Glaziou et al. (e47) investigated two doses of BB 10% versus IVER 0.1 mg/kg. No statistically significant difference in efficacy was found after 2 or 4 weeks (efigure 4). Five patients in the BB group reported increased pruritus. No adverse events (AEs) were reported in the IVER arm of the trial.
Bachewar et al. (e38) compared IVER 0.2 mg/kg to BB 25% applied on 2 consecutive nights and found no statistically significant difference in efficacy after 2 weeks (efigure 4). However, 44.4% and 24% of patients respectively underwent repeat treatment after one week. No AEs occurred.
Most trials did not report whether any patients underwent repeat treatment (efigure 4).
Sulfur ointment versus benzyl benzoate 25%
Gulati and Singh (e48) conducted a trial on the efficacy of sulfur versus BB 25%. Both ointments were to be applied 3 times, at intervals of 12 hours. After 14 days, no statistically significant difference was found in terms of the outcome parameter “clearance of lesions” (RR: 1.07; 95% CI: [0.99; 1.15]). Patients who still had lesions after day 10 underwent repeat treatment. AEs were not reported.