Figure 8.
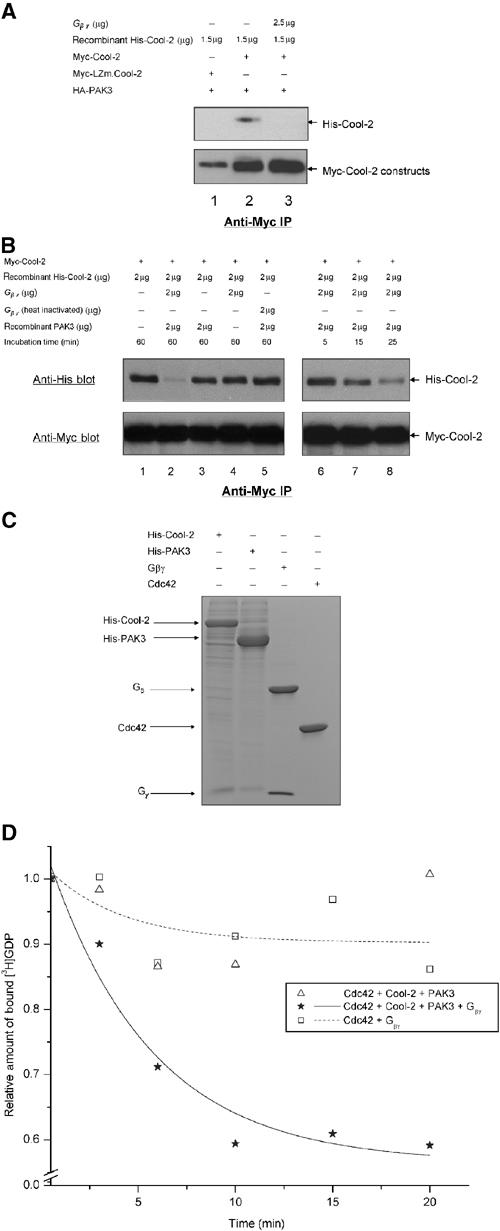
Gβγ helps to activate the Cdc42-GEF activity of Cool-2. (A) The ability of Myc-Cool-2 versus Myc-LZm.Cool-2 to dimerize was examined in the presence of PAK3, and in the presence and absence of bovine retinal β1γ1, by assaying the ability of insect cell-expressed His-Cool-2 to be co-immunoprecipitated with Myc-Cool-2 that was transiently expressed in COS-7 cells (see Materials and methods). (B) The time-dependent dissociation of the dimers formed between His-Cool-2 and Myc-Cool-2, upon the addition of retinal β1γ1 and insect cell-expressed His-PAK3, was assayed as described in Materials and methods. ‘Incubation time' refers to the time periods that β1γ1 and/or PAK3 were incubated with lysates expressing Myc-Cool-2 and containing His-Cool-2. (C) Coomassie blue staining of SDS–PAGE of purified protein preparations used in these experiments. Histidine-tagged Cool-2 and PAK3 were expressed and purified from insect cells (see Materials and methods), and GST-Cdc42 was expressed and purified from E. coli, with the GST tag being removed prior to the GEF assays. The retinal βγ subunit complex was isolated as previously described (Phillips et al, 1989). (D) E. coli-expressed Cdc42 (1 μg) was incubated with retinal βγ (5 μg) alone or with His-Cool-2 (4 μg) and His-PAK3 (2 μg) in the presence and absence of βγ, and then the dissociation of [3H]GDP from Cdc42 was assayed as described in the legend to Figure 2A.
