Figure 2.
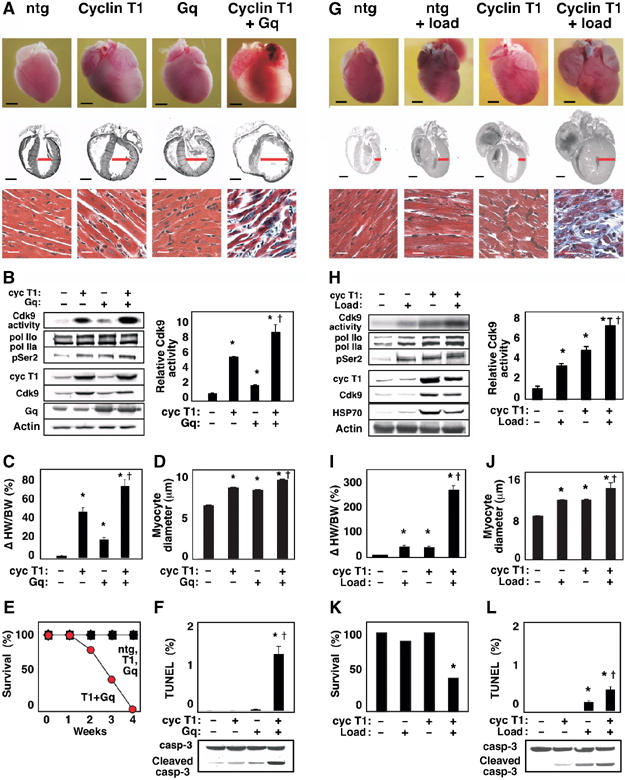
Cdk9 activation by cyclin T1 predisposes to heart failure, in concert with genetic or hemodynamic triggers of hypertrophy. (A–F) Cyclin T1 exacerbates the response to Gq. Mice were heterozygous for the indicated transgenes. Ntg, nontransgenic littermates. (A) Upper and middle rows, dilated cardiomyopathy (age, 2 weeks). Ventricular diameter is denoted in red. Bar, 2 mm. Lower row, Gomori-trichrome stain. Bar, 20 μm. (B) Synergistic activation of Cdk9 (immune complex kinase assay) and Ser2 phosphorylation of endogenous RNAPII (Western blotting). See Figure 1A for details. Right, mean±s.e. (C) Increased heart-weight-to-body-weight ratio. (D) Increased myocyte diameter. (E) Rapid mortality. (F) Increased apoptosis, detected as TUNEL-positive cardiomyocytes and caspase-3 cleavage. (B–D) *P<0.05 versus nontransgenic littermates; †P<0.05 versus cyclin T1 or Gq alone. (G–L) Cyclin T1 exacerbates the response to mechanical stress. (G) Upper and middle rows, ventricular and atrial enlargement in 3-month-old mice after partial transverse aortic occlusion for 21 days. Ventricular wall thickness is denoted in red. Bar, 2 mm. Lower row, Gomori-trichrome stain. Bar, 20 μm. (H) Synergistic activation of Cdk9 (immune complex kinase assay) and Ser2 phosphorylation of RNAPII (Western blot). (I) Increased heart-weight-to-body-weight ratio. (J) Increased myocyte diameter. (K) Increased mortality (21 days after occlusion). (L) Increased apoptosis. TUNEL stain and caspase-3 cleavage, 2 weeks after occlusion. (H–J) *P<0.05 versus nontransgenic control littermates; †P<0.05 versus cyclin T1 or load individually. N=5 for each condition shown (B–D, F, H–J, L).
