Figure 2.
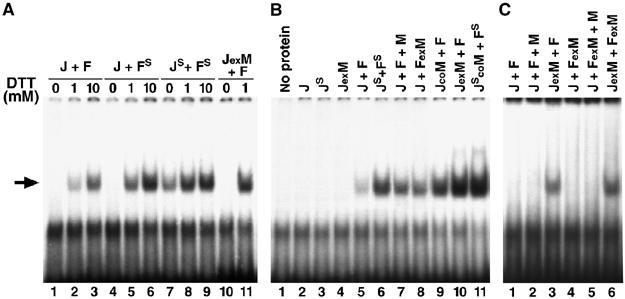
Effects of oxidation and MBF1 on the binding of D-Fos and D-Jun to an AP-1 site. Gel retardation assays were performed with Jun and Fos, each containing the single critical cysteine (J, F), and with the serine mutants (JS, FS). MBF1 was added separately (J+F+M), coexpressed with Jun (JexM) or Fos (FexM) from a bicistronic plasmid, or copurified from mixed E. coli cultures each expressing one protein (JcoM). The arrow shows the AP-1/DNA complex. (A) Binding of freshly purified proteins under oxidative (no DTT) or reducing conditions revealed that Drosophila AP-1 activity depends on the redox state of the critical cysteine residues in its DNA-binding domain. (B) Binding of freshly purified proteins in the presence of 1 mM DTT showed that weak AP-1 activity (lane 5) was greatly enhanced by coexpression of Jun with MBF1 (lane 10). Both Jun and Fos were required for the binding. (C) The assay conditions were as in (B) except that the proteins were aged for 5 days in solution at 4°C. No binding was observed unless MBF1 had been coexpressed with Jun.
