Figure 6.
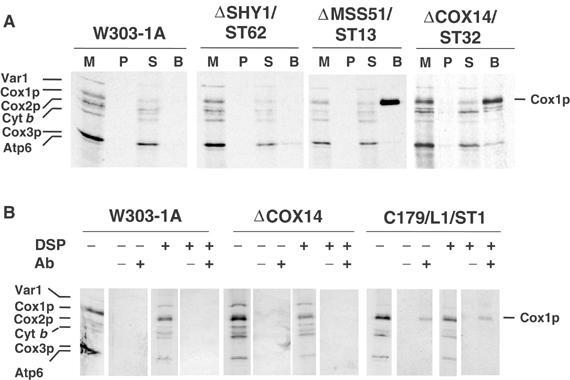
Cox14p and Mss51p interact with Cox1p. (A) Mitochondria were prepared from the wild-type W303-1A, a shy1 null mutant (ΔSHY1/ST62) with a chromosomally integrated plasmid expressing the Shy1p-GST fusion protein, an mss51 null mutant (ΔMSS51/ST13) with a chromosomally integrated plasmid expressing Mss51p-GST, and a cox14 null mutant (ΔCOX14/ST32) with a chromosomally integrated plasmid expressing Cox14p-GST. Mitochondria were labeled with [35S]methionine for 30 min and extracted with 1% lauryl maltoside, 1 M KCl, and 1 mM PMSF. The extract was clarified by centrifugation at 50 000 gav for 30 min and incubated with glutathione–Sepharose beads for 4 h at 4°C. After centrifugation at 1500 rpm for 5 min, the supernatant was collected and the beads were washed three times with PBS. Mitochondria (M) corresponding to 2 μg protein, equivalent volumes of the membrane pellet (P) after lauryl maltoside extraction and of the supernatant from the glutathione–Sepharose beads (S) were separated on a 17.5% polyacrylamide gel by SDS–PAGE. The amount of washed beads (B), however, corresponded to ∼500 μg of the starting mitochondria. (B) Mitochondria from W303-1A, the cox14 null mutant (ΔCOX14), and a cox14 point mutant transformed with a high-copy plasmid containing COX14 (C179/L1/ST1) were labeled for 30 min at 30°C in the presence of [35S]methionine. After a 5 min pulse, the samples were treated with the crosslinker DSP (+) or were mock-treated (−) as described (Hell et al, 2000). Immunoprecipitation of crosslinked adducts was performed using antiserum specific for Cox14p (+) and preimmune serum (−). Immunoprecipitates were analyzed by SDS–PAGE and autoradiography as in Figure 1.
