Figure 8.
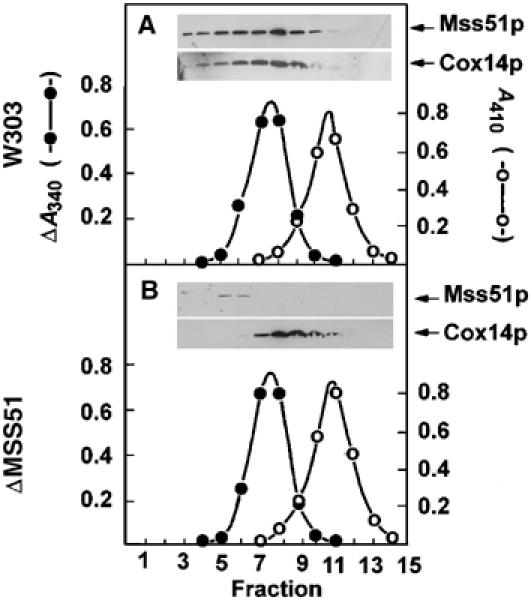
Sedimentation of Mss51p and Cox14p in sucrose gradients. (A) Mitochondria of the wild-type strain W303-1A were extracted at a protein concentration of 10 mg/ml with 1% lauryl maltoside, 20 mM Tris–HCl (pH 7.5), and 0.5 M KCl. The extract (0.4 ml) was mixed with 2.5 mg of hemoglobin and 60 μg lactate dehydrogenase and applied to 4.6 ml of a linear 7–25% sucrose gradient containing 10 mM Tris–HCl and 0.1% Triton X-100. Following centrifugation at 65 000 rpm in a Beckman SW65Ti rotor for 6 h, 14.5 fractions were collected, separated on a 12% polyacrylamide gel, transferred to nitrocellulose and probed with rabbit antiserum against Mss51p or Cox14p followed by a secondary goat peroxidase-conjugated antibody against rabbit IgG. Antibody–antigen complexes were visualized with the Super Signal reagent (Pierce Chemical Co., Rockford, IL). Hemoglobin (○- - -○) was estimated from absorbance at 410 nm and lactate dehydrogenase (⧫- -⧫) was assayed by measuring oxidation of NADH at 340 nm with pyruvate as the substrate. (B) Same as (A) except that the mitochondria were isolated from the mss51 null mutant aW303ΔMSS51 and the gradient was collected in 15 fractions.
