Lysates from CD8+ T cells transfer passive IL-17-mediated cellular immunity through elements of the TCR and S100; an opportunity to elucidate the structure, role, and therapeutic potential.
Keywords: transfer factor, cellular immunity, Tc17
Abstract
Cellular lysates from PPD+ donors have been reported to transfer tuberculin reactivity to naïve recipients, but not diphtheria reactivity, and vice versa. A historically controversial topic, the terms "transfer factor" and "DLE" were used to characterize the reactivity-transferring properties of lysates. Intrigued by these reported phenomena, we found that the cellular extract derived from antigen-specific memory CD8+ T cells induces IL-6 from antigen-matched APCs. This ultimately elicits IL-17 from bystander memory CD8+ T cells. We have identified that dialyzable peptide sequences, S100a9, and the TCR β chain from CD8+ T cells contribute to the molecular nature of this activity. We further show that extracts from antigen-targeted T cells enhance immunity to Staphylococcus aureus and Candida albicans. These effects are sensitive to immunization protocols and extraction methodology in ways that may explain past discrepancies in the reproducibility of passive cellular immunity.
Introduction
The ability of cells from immunized guinea pigs to transfer immunity against infectious agents to naïve guinea pigs established the presence of cellular immunity [1]. However, lysis of these cells reportedly did not fully negate their immunity-transferring properties [2]. The leukocyte lysate from human donors with a positive DTH test to tuberculin (PPD+) but a negative DTH test to diphtheria appeared to transfer tuberculin reactivity passively to subjects who were previously PPD− without impacting reactivity to diphtheria toxin [3]. Conversely, lysates from donors who were diphtheria toxin DTH+ but PPD− only enhanced recipient diphtheria toxin immunity but not tuberculin reactivity [3]. This effect was reportedly within the dialyzable, <8 kDa fraction [4] and was conserved between humans and other mammals [5, 6]. This dialyzed cellular lysate was entitled transfer factor [2] for its ability seemingly to transfer immunity. Uncontrolled case series reported a benefit in diseases, including tuberculosis and CMC, and a double-blind, placebo-controlled clinical trial showed protection against leukemia-related varicella zoster virus [7].
Subsequent to its initial description, numerous claims surrounding transfer factor emerged, including enhanced wound healing [8], improved leukocyte chemotaxis [9], and anti-neoplastic activity [10–12]. Reports following the discovery of the maternal transfer of antibodies [13] asserted that dialyzed bovine colostrum activated similar pathways as leukocyte extracts but failed to clarify if these findings were attributable to the same mechanisms [14]. The term DLE replaced transfer factor to better reflect the uncertainty of the lysate’s potential mechanisms. However, earnest investigation into passive cellular immunity has largely ceased in recent decades, likely a consequence of both experimental inconsistencies and waning therapeutic interest subsequent to the discoveries of HIV and hepatitis C. In approaching these historical reports with an open mind and modern techniques, we have confirmed transfer of immunity by DLE. We have further identified that DLE activity resides in antigen-specific CD8+ T cells and acts on antigen-loaded APCs to induce IL-6, which subsequently stimulates bystander memory CD8+ T cells. We show that the reportedly small, <8 kDa size of DLE activity is likely a reflection of past techniques and that instead, larger proteins are present, including bioactive TCR β chain and S100a9. We show that this passive cellular immunity is functional in both mice and humans and provides insights into the mechanistic basis and therapeutic potential of DLE.
MATERIALS AND METHODS
Mice
BALB/cJ, C57BL/6J, OT-I, OT-II, IFN-γ−/−, and C3H mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). TLR4−/− mice, also deficient in the LPS-inactivating enzyme acyloxyacyl hydrolase, were a kind gift from Robert Munford (NIAID, Bethesda, MD, USA). Stat3 transgenic [mut-Stat3, Tg(Stat3*)L1Alau] mice were a kind gift from John O’Shea (National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, MD, USA). IL-17A/F dko mice were a kind gift from Rachael Caspi (NEI, Bethesda, MD, USA). Mice were used between 7 and 12 wk of age. Experiments were performed in both male and female mice, but age and sex matched within each experiment. Experiments in BALB/c were repeated in mice purchased from Taconic Farms (Hudson, NY, USA). Mice from which antigen-naïve splenocytes were harvested and mice treated with DLE were also BALB/cJ, unless specified in figure legends. All experiments were done in compliance with the guidelines of the NIAID Institutional Animal Care and Use Committee.
Immunizations
An emulsion of OVA or BSA (Sigma, St. Louis, MO, USA) and CFA (BD Difco; Thermo Fisher Scientific, Waltham, MA, USA) was made so that both products had final concentrations of 2.5 μg/μl. Emulsions were generated in Micro-Mate glass syringes (Popper and Sons, Hyde Park, NY, USA). The emulsion (40 μl) was injected subcutaneously, just superior to the tail. Mice were then left for 3 wk. On d 21, a select number of mice were injected with 50 μg OVA or BSA into 1 hind paw, and 18–24 h later, the resultant difference in thickness between the injected paw and noninjected paw was measured to assure adequate sensitization (calipers; L. S. Starrett, Athol, MA, USA). To immunize with MRSA (USA300LAC strain from Frank DeLeo, NIAID), mice were infected subcutaneously with 1e8 CFU suspended in CFA on d 0. On d 7 and 14, mice were infected with 1e8 CFU suspended in IFA. On d 21, selected mice were footpad tested with 1e6 CFU of MRSA that was culture-verified killed after heating to 65°C for 45 min. For Candida immunizations, heat-inactivated C. albicans (SC5314 strain) was suspended to 2e8/ml in PBS and combined 1:1 for an emulsion with IFA. Candida:IFA (100 μl) was injected subcutaneously at the tail base. CFA:DLE was generated by injected mice with 40 μl of an emulsion of CFA and PBS for a final CFA concentration of 2.5 μg/ml.
DLE derivation
Only the mice that did not receive footpad testing were used to extract DLE. Spleens were harvested 21 d after the first immunization. Single-cell suspensions were made by homogenizing tissue through a 100 μm metal mesh (Trans World Pacific, Berkeley, CA, USA), suspended in 50 ml conical holders (Ammnra Creations, San Jose, CA, USA). Cell counts were obtained using Trypan blue (Lonza, Williamsport, PA, USA) and a manual cytometer (Marienfeld, Lauda-Königshofen, Germany), and splenocytes were resuspended in 5 ml sterile water (Lonza). Cells were frozen and then thawed using dry ice and 100% ethanol bath, alternating with a 37°C water bath. The freeze-thaw cycle was performed at least 7 times. The product was then placed into dialysis bags with an 8 kDa cutoff that had been boiled for 3 intervals of 20 min (Spectrum Labs, Rancho Dominguez, CA, USA) against 50 vol water. Dialysis was conducted for 24 h at 4°C under constant stir. Dialysis was repeated for an additional 24 h with an identical volume of water after the first amount was removed and frozen. Subsequent experiments using ultrafiltration were performed by filtering lysed splenocytes through a Centriprep-10K cutoff ultrafiltration unit (EMD Millipore, Billerica, MA, USA). After the second dialysis or after ultrafiltration, the contents from both dialysis sessions were combined in T225 flasks (BD Falcon; BD Biosciences, San Jose, CA, USA), frozen to −80°C, and then lyophilized (FreeZone 2.5; Labconco, Kansas City, MO, USA). Lyophilized remnants were resuspended in sterile water or DMEM (Thermo Fisher Scientific) to a cell-equivalent concentration, as indicated. DLE was injected into the intraperitoneal space in 500 μl at indicated concentrations. DLE was derived from BALB/cJ mice unless specified in the figure legends.
Test for antigen reactivity
Footpad testing was performed, as previously described [15]. Immunized or DLE-treated mice were challenged in the right hind paw with 50 μg OVA or BSA in 25 μl HBSS. Swelling was measured 18–24 h after footpad challenge in identical fashion to immunization. All swelling measurements for DLE-treated mice were done in blinded fashion as to the mouse receiving DLE or diluent.
Cell cultures
Murine DCs were derived from bone marrow. For each mouse, femur and tibia flushes were performed to obtain marrow material. For each mouse, the marrow was suspended in DC media consisting of 50 ml RPMI (Thermo Fisher Scientific) with 10% FBS (Thermo Fisher Scientific), penicillin/streptomycin (Thermo Fisher Scientific), and sodium pyruvate (Thermo Fisher Scientific); 25 ml was placed into each 150 mm tissue-culture dish (Techno Plastic Products, Trasadingen, Switzerland). Marrow cells were stimulated with GM-CSF (R&D Systems, Minneapolis, MN, USA) at 1 ng/ml, repeat supplementation was performed on d 3 by adding 25 ml media with GM-CSF at 2 ng/ml. On d 5, antigen pulsing was performed with OVA (20 ng/ml), e-OVA (20 ng/ml EndoGrade OVA; Hyglos, Bernried am Starnberger See, Germany), BSA (20 ng/ml), HKCA (5e7 CFU/ml), or heat-killed MRSA (5e6 CFU/ml). LPS (List Biological Laboratories, Campbell, CA, USA) supplementation for HKCA and BSA was matched to concentrations measured in OVA. Heat inactivation of MRSA was performed as previously described [16]. Human moDCs were generated as previously described [17]. Activation of DCs was performed by adding LPS at stated concentrations or by adding OVA contaminated with LPS at identical concentrations. DCs were suspended in media of DMEM, 10% FBS, penicillin/streptomycin, with nonessential amino acids (Thermo Fisher Scientific), and 2-ME (Thermo Fisher Scientific) for a concentration of 8e5 cells/ml. Splenocytes, cellular-sorted splenocytes, or PBMCs were suspended to 4e6 cells/ml and combined 1:1 with the DC or moDC. For in vitro assays, DLE was added in 1:10–1:1000 dilutions into the culture media for final concentrations of 1e2–2e8 ceq/ml or as indicated. For ex vivo stimulation, splenocytes from mice treated with DLE were harvested 24–48 h after DLE treatment and either suspended to 2e6 cells/ml and combined with antigen at stated concentrations or suspended to 4e6 cells/ml and combined 1:1 with DCs suspended at 8e5/ml or as indicated. Transwell experiments used identical concentrations separated by a 0.4 μm barrier (Costar, Corning, NY, USA). Experiments using antibody blockade used anti-IL-6 (AF-406-NA) at 0.02 μg/ml and anti-IL-1β (AF-401-NA) at 20 μg/ml from R&D Systems; these were placed into the DC compartment of the Transwell culture model. Experiments using antibody precipitation used TCR-α (H142; Santa Cruz Biotechnology, Dallas, TX, USA), TCR-β (H197; Santa Cruz Biotechnology), S100a9 (ab75478; Abcam, Cambridge, MA, USA), or isotype control (Santa Cruz Biotechnology). DLE was incubated overnight with 10 μg/ml antibody, and the following morning, antibodies were extracted using protein A magnetic beads (Thermo Fisher Scientific), per the manufacturer's instructions. Cytokines were measured by Bio-Plex (Bio-Rad Laboratories, Hercules, CA, USA), per the manufacturer's instructions. Values are displayed as change from diluent control when all cell types were identical across tested conditions, whereas both diluent and DLE-stimulated values are shown for experiments that differed in their cellular populations, such as with cells from knockout mice.
Human cell cultures
hDCs were obtained by as previously described [18]. hDCOVA and hDCHKCA were in an identical manner as murine bone marrow-derived DC. Autologous PBMCs were obtained 6–7 d after the DC culture was begun and cocultured with and without autologous PBMC. Cytokines were measured by Bio-Plex (Bio-Rad Laboratories), per the manufacturer's instructions. CFSE experiments were performed as previously described [19]. In brief, 5e4 monocytes were incubated 1:1 with CD3+ cells, labeled with 1 μM CFSE, per the manufacturer's instructions (Affymetrix, Santa Clara, CA, USA). Monocytes and T cells were sorted using SepMate and RosetteSep (Stemcell Technologies, Vancouver, BC, Canada), per the manufacturer's instructions.
LPS concentration
LPS concentration in OVA, colostrum, and DLE was performed using the limulus amebocyte lysate reaction kit (Hycult Biotech, Plymouth Meeting, PA, USA), per the manufacturer's instructions.
Skin and mucosal OVA challenges
For skin challenge of OVA, mice treated OVA:DLE were injected subcutaneously with 1 mg OVA in 100 μl of an emulsion with IFA. Pulmonary challenges were performed by intranasal inoculation with 1 mg OVA in 50 μl PBS. Seventy-two hours later, skin or lungs were harvested, organs were homogenized via TissueLyser I (Qiagen, Valencia, CA, USA), mRNA extracted via an RNeasy kit (Qiagen), and cytokine expression measured using TaqMan probes and PCR (detailed below).
MRSA skin infection
Infection, lesion measurement, CFU determination, and cytokine analysis were performed as previously described [16].
Mucocutaneous Candida disease model
Mouse challenges for early Candida responses were performed at 24 h after exposure to the SC5314 strain; challenges for d 5 responses were performed using the Y72 strain. Each challenge was performed as previously described [20].
Murine cell sorting
Cells for mice and human were sorted using negative-selection CD8 microbeads (Miltenyi Biotec, Gladbach, Germany) on an autoMACS Pro (Miltenyi Biotec). More detailed murine cell sorting was performed by staining with CD3-PE (145-2C11), CD4-FITC (GK1.5), CD8-allophycocyanin (53-6.7), or CD8-allophycocyanin with CD44-PE (IM7), and CD62L-FITC (MEL-14), per the manufacturer's instructions (eBioscience, San Diego, CA, USA). Cells were separated on a FACSAria III (BD Biosciences). MHC-I tetramers coupled to PE were obtained from the U.S. National Institutes of Health Tetramer Facility (Emory University, Atlanta, GA, USA).
RT-PCR
RNA was prepared using TaqMan One-Step RT-PCR Master Mix instructions (Thermo Fisher Scientific). All samples were analyzed on a 7500 Fast Real-Time PCR System (Thermo Fisher Scientific), as previously described [16, 21]. Mouse primers were all purchased from Thermo Fisher Scientific: IL-17A (Mm00439619_m1), IL-17F (Mm00521423_m1), IL-22 (Mm00444241_m1), IFN-γ (Mm01168134_m1), IL-1β (Mm01336189_m1), IL-6 (Mm00446190_m1), IL-23a (Mm00518984_m1), TNF (Mm00443258_m1), and S100a9 (Mm00656925_m1). mRNA expression from infected skin was compared with uninfected skin from naïve mice or an infected wild-type control as indicated, using the cycle threshold difference method.
RNA-seq
1e6 DCOVA were incubated with 1e6 ceq of indicated DLE for 18 h. mRNA was extracted via the RNeasy kit (Qiagen). DC stimulation was repeated 3 times on 3 different occasions. TruSeq libraries were prepared from 4 μg total RNA input, with almost all samples having RNA integrity number > 9.0, using the Illumina Stranded mRNA kit. Sequencing was performed using the Illumina NextSeq500 instrument, reading 75 bases on each paired end. Yield averaged 32 million read pairs/sample. Reads were aligned to the mouse mm10 genome with TopHat2 and mapped to the mouse RefSeq transcriptome (2015-0804) with the expectation–maximization algorithm in Partek Flow (Build 4.0.15.0702; Partek, St. Louis, MO, USA). Read counts/gene were normalized as reads/million (aligned) reads by sample, converted to log2 (+1 shifted), and normalized by the upper quantile across samples. ANOVA was performed on the normalized counts to test mRNA expression differences in DCs after addition of DLEs or diluent control. P values were adjusted for multiple testing using the false discovery rate method with a cutoff of 0.05. Normalizations and statistical analysis were performed primarily in JMP Genomics 7.0 (SAS Institute, Cary, NC, USA).
MS and protein identification
Identification of proteins was performed on reduced and alkylated, trypsin-digested samples, prepared by standard MS protocols. The supernatant and 2 washes (5% formic acid in 50% acetonitrile) of the gel digests were pooled and concentrated by SpeedVac (Labconco) to dryness, directly in 200 µl polypropylene auto-sampler vials (SUN-SRi, Rockwood, TN, USA). The recovered peptides were resuspended in 5 µl solvent A (0.1% formic acid, 2% acetonitrile, and 97.9% water).
Before MS analysis, the resuspended peptides were chromatographed directly on column, without trap cleanup. The bound peptides were separated at 500 nl/min, generating 80–120 bar pressure, using an aQ C18 reverse-phase media (3 µm particle size and 200 µm pore), packed in a pulled tip, nano-chromatography column (0.100 mm inner diameter × 150 mm length) from Precision Capillary Columns (San Clemente, CA, USA). The chromatography was performed in-line with an LTQ-Velos Orbitrap mass spectrometer (Thermo Fisher Scientific), and the mobile phase consisted of a linear gradient prepared from solvent A and solvent B (0.1% formic acid, 2% water, and 97.9% acetonitrile) at room temperature. Nano liquid chromatography-tandem MS was performed with an Easy-nLC II multidimensional liquid chromatograph and temperature controlled Ion MAX Nanospray source (Thermo Fisher Scientific) in-line with the LTQ-Velos Orbitrap mass spectrometer.
Data were searched using the Software for Protein Identification from Sequence Tags Containing De Novo Sequencing homology search approach in PEAKS v7.5 (Bioinformatics Solutions, Waterloo, ON, Canada). The database search was performed against human and mouse proteins found in the UniProtKB/Swiss-Prot database and proteins found in the Common Repository of Adventitious Proteins database (The Global Proteome Machine; theGPM.org). Peptides were filtered at a 0.5% peptide FDR, as determined by the use of a reverse decoy database. Protein assignments required a 2 peptide/protein minimum.
Peptide synthesis
Synthesized peptides were purchased commercially through AnaSpec (Fremont, CA, USA) at >90% purity.
ELISA
OVA:DLE or DLE from antigen naïve mice was serially diluted in water, as indicated, and incubated in 96-well immunoplates (BD Falcon; BD Biosciences) overnight at 4°C to replace the capture antibody in a standard sandwich ELISA. After blocking with 10% FBS (Thermo Fisher Scientific), 50 μg/ml OVA or BSA (Sigma) was added to the wells and detection performed with a secondary antibody (Bethyl Laboratories, Montgomery, TX, USA). Standard curves were generated by placing the block into wells incubated overnight with only PBS. Serial dilution of OVA or BSA was performed and placed into the blocked well after 3 washes. The signal for bound OVA and BSA was analyzed against expected value for the added concentration of OVA or BSA. In select experiments, synthesized peptides were used in place of DLE or capture antibodies. In other experiments, OVA or synthetic peptides for the MHC-I epitope (OVA257–264) or MHC-II epitope (OVA323–339; both purchased from AnaSpec) were incubated at 100 μg/well in 96-well immunoplates overnight at 4°C. After blocking with 10% FBS and washing 3 times, 2e6 ceq of OVA:DLE was added to each well. After overnight incubation at 4°C, the OVA:DLE was removed and was incubated in 96-well immunoplates overnight at 4°C to replace the capture antibody, as described previously. For TCR chain and S100a9 analysis, 96-well immunoplates were incubated overnight at 4°C with 1e5 ceq of indicated DLE preparations. Rabbit anti-mouse primary antibodies included TCR-α (H142; Santa Cruz Biotechnology), TCR-β (H197; Santa Cruz Biotechnology), S100a9 (ab75478; Abcam), or isotype control (Santa Cruz Biotechnology). Chicken anti-rabbit IgG conjugated to HRP was used as the secondary antibody (sc-516087; Santa Cruz Biotechnology).
Statistics
Means were compared using 2-tailed unpaired t test or ANOVA for comparison of multiple samples with Prism software (GraphPad Software, San Diego, CA, USA).
Study approval
All animal experiments were conducted under approved Office of Animal Care and Use procedures. All human sample collection and processing were performed under IRB-approved clinical trials, and all subjects gave full consent to sample collection. All participants provided their written consent to the research protocol, and IRB consent was obtained before blood collection.
RESULTS
DLEs induce antigen-specific reactivity in mice
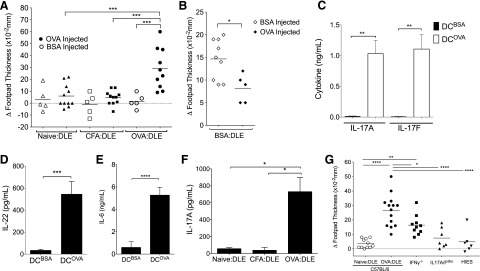
To generate DLE, BALB/c mice (unless specified in the figure legends) were immunized with an emulsion of OVA and CFA, as described in prior literature [22]. Splenocytes were later harvested, lysed by freeze-thaw cycling, and then dialyzed in bags designed to limit the molecular size to <8 kDa. This OVA-targeted, dialyzed, splenic lysate (OVA:DLE) was injected intraperitoneally into OVA-naïve mice. Twenty-four hours later, the DLE-treated mice underwent standard footpad testing. Mice treated with OVA:DLE had significantly greater footpad swelling upon OVA challenge but not when footpads were injected with BSA (Fig. 1A). Splenocyte extracts prepared similarly from mice exposed only to CFA (CFA:DLE) or from mice that were antigen naïve (naïve:DLE) did not enhance footpad swelling (Fig. 1A). Similar, but less robust, results were seen in subsequent experiments using DLE from mice immunized with BSA in CFA (BSA:DLE; Fig. 1B), further suggesting that the sensitization was antigen specific.
Figure 1. Cellular lysates transfer antigen-specific immunity.
Mice were injected with 2e6–5e6 ceq of DLE from antigen-naïve mice (Naïve:DLE), mice injected with CFA (CFA:DLE), or mice immunized with an emulsion in CFA of either BSA or OVA (BSA:DLE or OVA:DLE). (A and B) Twenty-four to 36 h after DLE (5e6 ceq) treatment, mice underwent footpad testing with OVA or BSA. The change in footpad thickness (Δ) compared with the opposing, noninjected footpad was measured in a blinded fashion, 18–24 h later. Individual symbols represent 1 mouse. (C–E) Twenty-four to 36 h after OVA:DLE (2e6 ceq) treatment, splenocytes were harvested and challenged ex vivo with DCOVA or DCBSA. Supernatants were harvested 3 d later, and concentrations of IL-17A (C), IL-22 (D), and IL-6 (E) were measured. (F) Twenty-four to 36 h after DLE (2e6 ceq) treatment, splenocytes were harvested from individual mice and processed into single-cell suspensions. The splenocytes underwent ex vivo challenge by coculturing 2e6 whole splenocytes with 4e5 bone marrow-derived DCOVA in a total of 1 ml media. Supernatants were harvested 3 d later, and IL-17A concentrations were measured. (G) Footpad testing with OVA, as in A, for wild-type (C57BL/6) versus IFN-γ knockout (IFN-γ−/−), a Stat3 transgenic mouse model of HIES, and IL-17A/F dko mice. Data shown are representative of 3 or more independent experiments, each using at least 5 mice/group (A–F) or a combination of 2 independent experiments using 4–7 mice/group (G) and displayed as means (A, B, and G) or means ± sem (C–F). ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, as determined by ANOVA (A, F, and G) or Student’s t test (B–E).
DLE induces antigen-specific ex vivo activation of the IL-17 pathway
Given that DTH responses are T cell driven [23], we next tested for a T cell cytokine response that could be used as an immunologic surrogate for footpad swelling. Ex vivo stimulation with DCOVA but not DCBSA induced IL-17A and IL-17F (Fig. 1C) and IL-22 (Fig. 1D) from splenocytes harvested from OVA:DLE-treated mice. Cytokines known to modulate IL-17 and IL-22 were similarly induced in an antigen-specific manner, including IL-6 (Fig. 1E), IL-1β, and TNF-α but not IFN-γ (Supplemental Fig. 1A–C). DCOVA stimulation of splenocytes from mice treated with CFA:DLE or naïve:DLE did not elicit this IL-17A response (Fig. 1F). Ex vivo activation of the IL-17 axis was also seen by adding OVA directly to splenocytes of OVA:DLE-treated mice (not shown).
DLE footpad swelling is dependent on IL-17A/F
Footpad swelling has traditionally been viewed as an IFN-γ-mediated process [24]. To assess a possible connection between footpad swelling and the IL-17 pathway induced by DLE, we repeated the footpad experiments in IFN-γ−/− and IL-17A/F dko (IL-17A/Fdko) mice. We also used a transgenic mouse model for a Stat3HIES [mut-Stat3, Tg(Stat3*)L1Alau] and is thus deficient in IL-17 production [25]. We found complete loss of footpad reactivity in response to OVA:DLE in both the Stat3HIES transgenic and IL-17A/Fdko mice (Fig. 1G). Footpad responses were significantly diminished but not abolished in the IFN-γ−/− mice (Fig. 1G), suggesting partial dependence on IFN-γ and complete dependence on IL-17 signaling.
DLE stimulates antigen-specific IL-17 production from antigen-naïve splenocytes
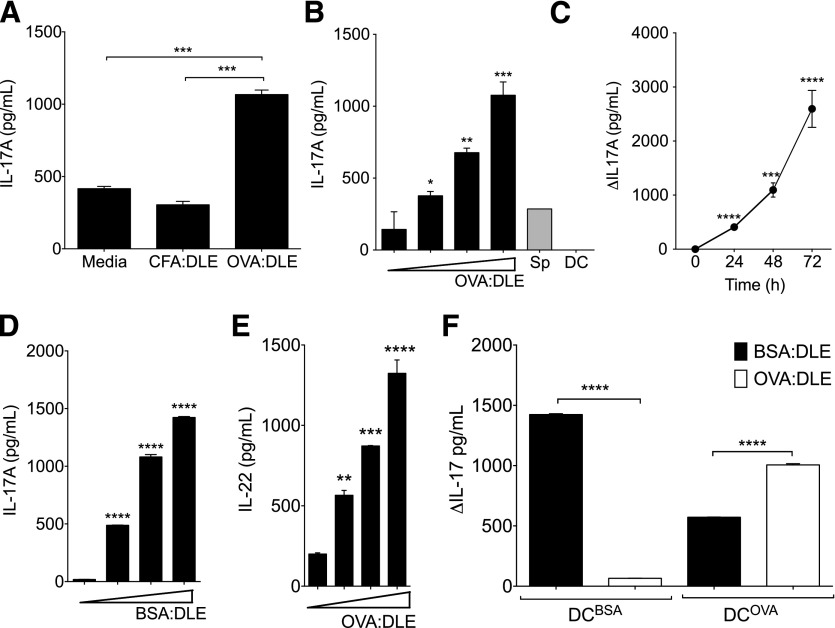
To evaluate if the ability of DLE to induce antigen-specific responses in vivo could be recapitulated in vitro during interaction of APCs with naïve splenocytes, we added DLE to cocultures of DCOVA and splenocytes from OVA-naïve mice. Addition of OVA:DLE to the media during coculture enhanced IL-17A production, whereas addition of CFA:DLE did not (Fig. 2A). The effects of OVA:DLE on IL-17A exhibited a dose (Fig. 2B)- and time (Fig. 2C)-dependent pattern. Addition of OVA:DLE to either splenocytes or DCOVA in isolation did not induce IL-17A, indicating that both cell types were required (Fig. 2B). Similar dose-dependent IL-17A induction was seen when adding BSA:DLE to cocultures of DCBSA and BSA-naïve splenocytes (Fig. 2D). Induction of IL-6 was also seen with both OVA:DLE and BSA:DLE stimulations (Supplemental Fig. 1D and E). Additional analysis showed induction of IL-22 (Fig. 2E), IL-1β, and TNF-α (Supplemental Fig. 1F and G) but only modest induction of IL-10 (Supplemental Fig. 1H). Inhibitory effects were seen on IFN-γ, IL-2, and IL-4 (Supplemental Fig. 1I–K). Furthermore, cocultures of DCBSA and splenocytes produced more IL-17A (Fig. 2F) and IL-6 (Supplemental Fig. 1L) in response to BSA:DLE than OVA:DLE. DCOVA conditions produced significantly more IL-17A (Fig. 2G) and IL-6 (Supplemental Fig. 1L) with both BSA:DLE and OVA:DLE treatment, but the response remained significantly larger in the antigen-matched condition, further suggesting antigen specificity.
Figure 2. DLE stimulates antigen-specific IL-17 production from antigen-naïve splenocytes.
Whole splenocytes (2e6) were harvested from OVA-naïve mice and cocultured with DCOVA (4e5) in 1 ml total media. (A) 1e5 ceq of DLE from mice immunized with CFA and OVA (OVA:DLE) or CFA alone (CFA:DLE) was added to the culture media. Supernatants were harvested after 72 h and analyzed for IL-17A. (B) Dose-response curve for OVA:DLE (0, 1e4, 1e5, and 1e6 ceq) added to cocultures (black bars) or splenocytes (Sp) or DC alone (gray bars; 1e6 ceq). IL-17A measured after 72 h of incubation. (C) 1e6 ceq of OVA:DLE added to cocultures. IL-17A measured at 24 h increments and depicted as change (Δ) versus time-matched media control. (D) Titrated doses (0, 1e4, 1e5, and 1e6 ceq) of DLE derived from mice immunized with CFA and BSA (BSA:DLE) were added to cocultures of BSA-naïve splenocytes and DCBSA. Supernatant IL-17A was measured after 72 h. (E) IL-22 induced from 1e4–1e6 ceq OVA:DLE added to cocultures as in B. (F) 1e6 antigen-naïve splenocytes were cocultured with 4e5 DCBSA or DCOVA in 1 ml total media. 1e6 ceq of either OVA:DLE or BSA:DLE was added; IL-17A concentrations at 72 h measured and depicted as the change from media control. Antigen-naïve splenocytes were assayed independently from at least 3 mice/group. Data shown are representative of 3 or more independent experiments and displayed as means ± sem. Significance shown versus media control baseline or as indicated. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, as determined by ANOVA.
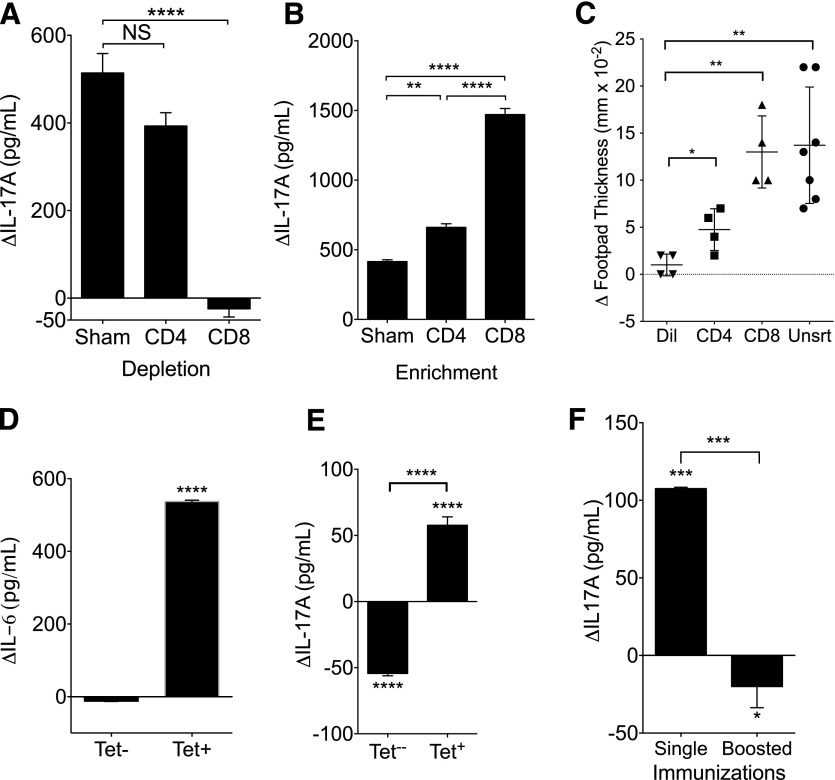
Antigen-specific CD8+ T cells are responsible for DLE induction of IL-17A
We next sought to identify the cellular source of the DLE effects. We immunized mice with OVA and CFA as before, but rather than extracting from whole splenocytes, we sorted the cellular fractions before the extraction. Depletion of the CD8+ cells negated the ability of cellular lysates to induce IL-17A from cocultures of splenocytes and DCOVA (Fig. 3A). Depletion of the CD4+ fraction had no significant impact on IL-17A production (Fig. 3A). Likewise, CD8+ enrichment enhanced IL-17A induction, whereas CD4+ enrichment had significantly less effect (Fig. 3B). Injection of DLE derived from splenocytes enriched for CD8+ T cells led to greater ex vivo production of IL-17A (Supplemental Fig. 2A) and enhanced footpad responses (Fig. 3C). Sorting of CD8+ T cells by binding to OVA-MHC-I tetramer before DLE extraction revealed that tetramer-positive CD8+ DLE enhanced IL-6 production from DCOVA (Fig. 3D) and IL-17A from cocultures of splenocytes and DCOVA (Fig. 3E), whereas DLE sourced from tetramer-negative cells did not induce either cytokine (Fig. 3D and E). Tetramer-positive cells represented 7.7% of the overall CD8+ population and 4.1% of the total CD3+ population (not shown). Taken together, these data suggest that the antigen specificity of DLE may be derived from the antigen specificity of the source CD8+ T cell. Of note, use of a booster immunization before DLE extraction (OVA:CFA injected on d 0; additional injection on d 21; extraction on d 42) strengthened the immunization, as measured by footpad response (not shown). However, boosting negated the ability of the subsequent cellular extract to enhance IL-17A production (Fig. 3F), indicating the potential for disassociation of the traditional DTH footpad response from DLE activity.
Figure 3. DLE effects are produced by antigen-specific CD8+ T cells.
(A–D) C57BL/6 mice were immunized with an emulsion of OVA and CFA; 3 wk later, cells were sorted by beads (A and B) or flow cytometry (C–E), and then DLE was extracted from sorted populations. Change (Δ) in induction of IL-17A from DCOVA:splenocyte cocultures by depleted (A) or enriched (B) DLE. (C) Mice were injected with diluent (Dil) or 1e6 ceq of DLE derived from unsorted (Unsrt) or sorted (CD3+/CD4+ or CD3+/CD8+) splenocytes. Footpad testing to OVA was performed 24 h after DLE treatment. (D and E) Splenocytes from mice immunized to OVA and CFA were sorted for CD3+/CD8+ and then subdivided by staining of the MHC-I OVA tetramer. 1e5 whole splenocytes were cocultured with 2e4 DCOVA in 150 μl total media, along with 5e4 ceq of tetramer-positive CD8+ DLE (Tet+) or 1e5 ceq of CD8+/tetramer negative (Tet−) DLE. Change (Δ) from diluent control for IL-6 at 24 h (D) and IL-17 at 72 h (E) is shown. (F) Mice were immunized with OVA in CFA on d 0 and 21. DLE was extracted on d 42 as before (Boosted). 1e6 ceq of boosted OVA:DLE or OVA:DLE from mice immunized only once (Single) was added to the media of 2e5 splenocytes cocultured with 4e4 DCOVA; change in IL-17A concentration versus diluent control at 72 h is shown. Antigen-naïve splenocytes were assayed independently from at least 3 mice/group. Data shown are representative of 3–4 independent experiments and displayed as means ± sem. Significance shown versus media control baseline or as indicated. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, as determined by ANOVA (A–C) or Student’s t test (D–F). NS, Not significant.
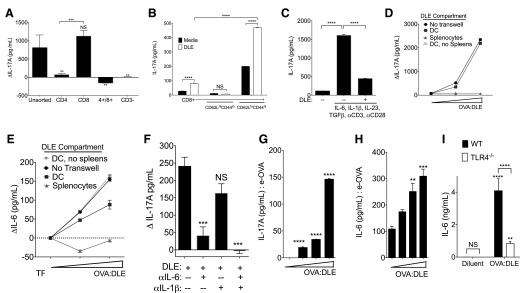
DLE stimulates memory Tc17 cells via activation of DCs
To evaluate the cellular source of splenocyte IL-17A production after DLE exposure, we sorted splenocytes before coculturing them with DCOVA and OVA:DLE. CD8+ T cells produced IL-17A when cultured with DCOVA and OVA:DLE, whereas CD4+ T cells or double-positive T cells did not (Fig. 4A). Further sorting revealed that memory CD62Llo/CD44hi CD8+ T cells were the source of the IL-17A production (Fig. 4B). Interestingly, although whole splenocyte (Supplemental Fig. 1I) and total CD8+ T cell populations (Supplemental Fig. 2B) did not produce more IFN-γ in response to DLE treatment, the memory CD62Llo/CD44hi CD8+ population did respond with greater IFN-γ production in addition to IL-17A (Fig. 4B and Supplemental Fig. 2B). Conditions that induce IL-17 production from CD8+ T cells (Tc17) and CD4+ T cells (Th17) are similar [26]. Like Th17 induction, Tc17 cells require IL-6 and TGF-β [27, 28], as well as the transcription factors STAT3, retinoic acid-related orphan receptor γ T, and IFN regulatory factor 4 [27]. Yet, Th17 induction was absent, despite induction of IL-1β and IL-6 (Supplemental Fig. 1A and D) and the presence of TGF-β (not shown). OVA:DLE directly inhibited CD4+ production of IL-17A when added alongside Th17 induction conditions (Fig. 4C).
Figure 4. DLE activates DCs to stimulate memory Tc17 cells.
Splenocytes were harvested from OVA-naïve mice and then sorted by flow cytometry before being placed into coculture with DCOVA in 1 ml total media. 2e6 ceq of OVA:DLE was added to the media. (A) 1e6 CD3+ splenocytes sorted by CD4 and CD8 positivity cocultured for 72 h with 4e5 DCOVA. Differences (Δ) in IL-17A from media control without DLE are shown. (B) IL-17A levels at 72 h from 2e5 CD3/8+ cells subsorted by CD62L and CD44 expression before coculture with 4e4 DCOVA in the presence of 2e6 ceq of OVA:DLE. (C) 2e6 CD3+/CD4+ cells were cultured under Th17 conditions, with and without 2e6 ceq of OVA:DLE. IL-17A at 72 h is shown. (D–F) 2e6 whole splenocytes were cultured with 4e5 DCOVA separated by a semipermeable Transwell membrane. 1e6–5e6 ceq of OVA:DLE was added to either the DC (above the transwell membrane) or splenocyte compartment (below the membrane). Controls included cocultures without the Transwell separator and DCOVA cultured in isolation (DC, no spleens). Change vs. diluent control for IL-17A after 72 h (D) and IL-6 at 24 h (E) are shown (dotted line in E represents no change from diluent control). (F) Antibodies against IL-6 and/or IL-1β were added to the DC compartment before coculture with whole splenocytes and OVA:DLE; change in 72 h values of IL-17A versus media control without DLE are shown. (G and H) IL-17 (G) and IL-6 (H) induced by OVA:DLE (0, 1e4, 1e5, and 1e6 ceq) from cocultures of whole splenocytes and DCe-OVA. (I) IL-6 production from 5e5 DCOVA derived from C57BL/6 wild-type (WT) mice or TLR4−/− mice was incubated for 18 h with 1e6 ceq of OVA:DLE or diluent control. Antigen-naïve splenocytes were assayed independently from at least 3 mice/group. Data shown are representative of 3–4 independent experiments and displayed as means ± sem. Significance shown versus media control baseline or as indicated. ****P < 0.0001, ***P < 0.001, **P < 0.01, as determined by ANOVA.
To evaluate if DLE activity required direct DC-to-T-cell contact, we repeated the culture conditions using a semipermeable Transwell separator and collected the combined media from both chambers. Incubation of DLE with the DCOVA compartment, but not the splenic compartment, induced IL-17A (Fig. 4D), indicating that direct contact between them was not required. Although both DC and splenocytes were required for IL-17A induction (Fig. 2B), OVA:DLE induced IL-6 from DCOVA even in the absence of splenocytes (Fig. 4E). The induction of IL-17A was inhibited by IL-6 neutralization and further reduced by concurrent blockade of IL-1β and IL-6 (Fig. 4F). These results suggest that DLE acted directly on DCOVA to induce IL-6, but resultant splenocyte IL-17A production did not require direct cell contact with DCs. Of note, DLE did not induce DC responses when placed in the splenocyte chamber, indicating its inability to cross the Transwell membrane. As the splenocytes were from OVA-naïve mice, our results also indicate that the DC is the source of the observed antigen specificity and that antigen-matched T cells are not required for IL-17A production.
The routine contamination of OVA with LPS potentially confounded our DC stimulation assays. Pulsing of the DC with e-OVA (EndoGrade OVA) rather than standard OVA reduced the magnitude of, but not capacity for, IL-17A induction (Fig. 4G) and IL-6 (Fig. 4H). Additionally, there was no detectable LPS in our DLE preparations (not shown). Likewise, DLE activated DCs derived from mice deficient in the LPS receptor TLR4−/− (Fig. 4I), as well as the TLR4 mutant C3H strain of mice (Supplemental Fig. 2C). However, IL-6 production in TLR4−/− and C3H DCs was significantly less than wild-type controls (Fig. 4I and Supplemental Fig. 2C). Both TLR4−/− and C3H splenocytes showed no defect in IL-17A production versus wild-type splenocytes when cocultured with wild-type DCOVA (Supplemental Fig. 2D), further suggesting that the DC is the cellular mediator of DLE responses. Together, these data indicate that antigen-specific effects of DLE were enhanced by, but did not require, LPS activation of the DC.
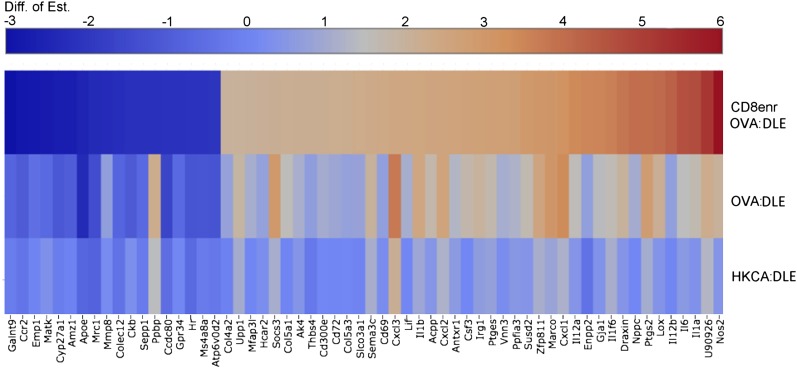
Our cytokine findings were supported by RNA-seq analysis of DCOVA that were stimulated with OVA:DLE, CD8+-enriched OVA:DLE or extracts from mice immunized against HKCA (HKCA:DLE). Consistent with our data, suggesting an antigen-specific CD8+ source of the immune activation, OVA:DLE induced significant IL-6 production that was further enhanced by CD8+ enrichment (Supplemental Fig. 2E). In contrast, incubation of DCOVA with the antigenically unmatched HKCA:DLE only marginally enhanced IL-6 levels compared with control (Supplemental Fig. 2E). We filtered mRNA expression data to select genes that were significantly impacted by OVA:DLE relative to the antigen-independent activation by HKCA:DLE and further enhanced by CD8+ enrichment. This analysis identified differential expression of genes in common DC pathways, including IL-1α, IL-1β, IL-6, lipoxygenase, cyclooxygenase 2 (PG-endoperoxide synthase 2), and Nos2 (Fig. 5 and Supplemental Material). Additionally, DLE treatment enhanced expression of chemokines, including CXCL1, CXCL3, and CXCL7 (pro-platelet basic protein), and the uncharacterized, putative, TNF resistance-related protein (U90926; Fig. 5 and Supplemental Material). Taken together, these results indicate that DLE acts on DCs that are presenting its relevant-matched antigen to induce IL-6 and other cytokines. This DC cytokine response drives IL-17A production from memory CD8+ cells, which are not necessarily specific for the DLE antigen and do not require direct contact with the DC.
Figure 5. DLE activates inflammatory genetic pathways in DCs.
RNA-seq heat map for 1e6 DCOVA incubated for 18 h with 1e6 ceq of indicated DLE compared with diluent control. Selected for display were genes that had statistically significant differential expression [difference of estimation (Diff. of Est.); log2 ratio > 2 in the hypothesized direction, and FDR < 0.05] for all 3 tests: OVA:DLE versus HKCA:DLE, OVA:DLE versus diluent, and CD8-enriched (CD8enr) OVA:DLE versus OVA:DLE. The table of log2 ratio values was sorted, according to the value for CD8enr OVA:DLE versus OVA:DLE, and colorized as indicated in the legend. Data shown are combined from 3 independent experiments; significance determined by ANOVA.
DLE impacts mucosal and cutaneous immunity against pathogens
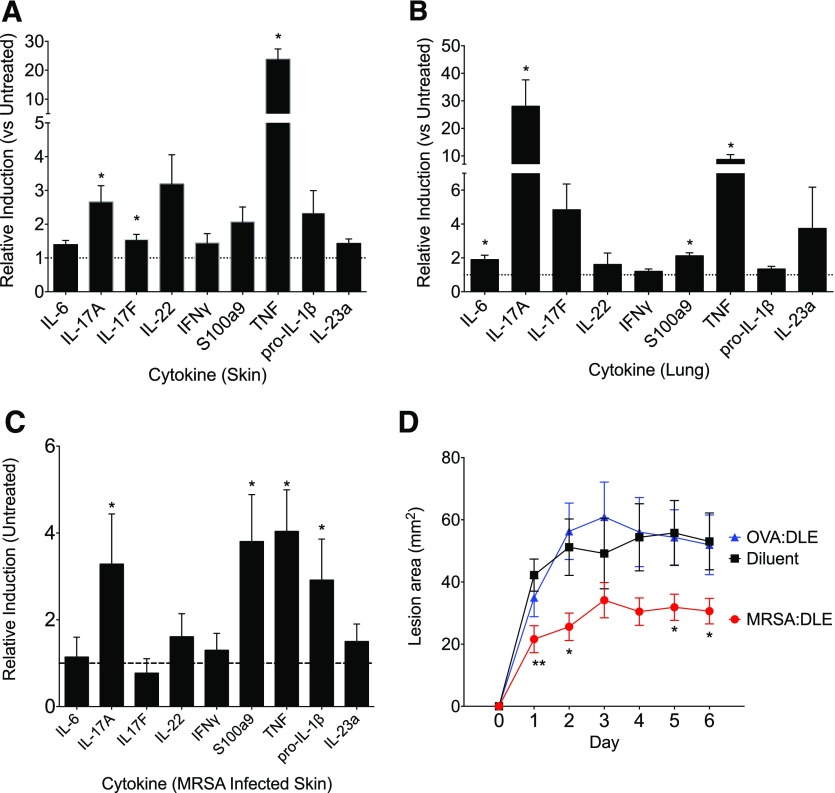
We next evaluated the effects of DLE on in vivo challenge models. We hypothesized that DLE could contribute to mucosal immunity, given the importance of IL-17A at the mucosa [29] and the influence of DLE on memory CD8+ T cells, the predominant resident T cell in human mucosa [30]. First, to assess the ability of DLE to induce antigen-specific mucosal responses, mice treated with OVA:DLE were challenged with OVA subcutaneously or intranasally and then evaluated for tissue cytokine responses. OVA:DLE significantly enhanced the IL-17A transcript levels in both skin (Fig. 6A) and lung (Fig. 6B). We then infected mice with MRSA subcutaneously after treatment with DLE taken from mice serially infected with MRSA (MRSA:DLE). MRSA:DLE-treated mice had enhanced skin transcript levels for IL-17A, pro-IL-1β, TGF-β, and S100a9 (Fig. 6C) after challenge. Consistent with this induction of responses relevant for the control of S. aureus [31], MRSA:DLE-treated mice had smaller lesion sizes (Fig. 6D) and a nonsignificant trend toward reduction of CFUs (Supplemental Fig. 3A). MRSA:DLE enhanced IL-17A production in a dose-dependent manner when added to cultures of splenocytes and heat-killed DCMRSA (Supplemental Fig. 3B). Past hDLE studies commonly targeted Candida [32], and DLE prepared from mice immunized with HKCA (HKCA:DLE) induced IL-6 from DCHKCA (Supplemental Fig. 3C) and IL-17 from coincubated, naïve splenocytes (not shown). However, in a mouse model of oral CMC, HKCA:DLE suppressed mucosal production of IL-17A and nonsignificantly worsened fungal control (Supplemental Fig. 3D and E). This may reflect the predominance of CD4+ T cell and innate sources of protective IL-17A in this mouse model [33], some of which may be inhibited by DLE, as suggested by our data on Th17 inhibition (Fig. 4C). These in vivo studies confirm the ability of DLE to induce mucosal responses, although the clinical outcome is influenced by pathogen- and model-specific factors.
Figure 6. DLE impacts mucosal and cutaneous immunity against pathogens.
(A and B) Mice were injected with 2e6 ceq of OVA:DLE. Twenty-four hours later, mice were challenged via injection of 1 mg OVA in IFA either subcutaneously (A) or intranasally (B). Seventy-two hours later, skin or lungs were harvested, and mRNA was extracted. Relative inductions of various cytokines compared with untreated (dotted, horizontal lines) normalized to GAPDH for skin (A) and lung (B). (C and D) Mice were injected with 2e6 ceq of DLE from mice serially infected with MRSA (MRSA:DLE). Twenty-four hours later, mice were challenged subcutaneously with 1e7 CFUs of MRSA. (C) d 6, relative inductions in lesional mRNA for cytokines compared with infected but DLE-untreated mice (dotted, horizontal line) normalized to GAPDH. (D) Lesional area measured daily for mice infected with 1e7 CFU of MRSA, 24 h after treatment with diluent, MRSA:DLE, or OVA:DLE. Data shown are representative of 3 or more independent experiments, each using at least 5 mice/group, and displayed as means (A and B) or means ± sem (C and D). **P < 0.01, *P < 0.05, as determined by ANOVA.
DLE induces IL-17 pathways in human cells
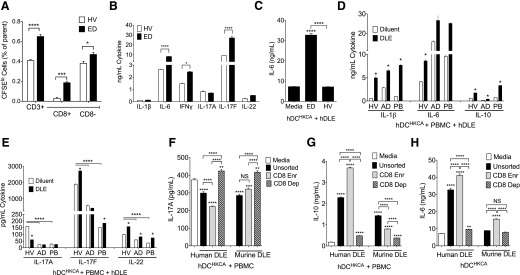
We next investigated the effects of DLE derived from human cells. As our results in mice suggested that DLE was produced by antigen-specific CD8+ T cells, we hypothesized that a given donor’s DLE would reflect their T cell repertoire. Therefore, to attempt enrichment for specificity against Candida, a common target of past hDLE studies [32], we derived DLE from a healthy cohabitant of 2 patients with CMC, resulting from autosomal-dominant, GOF STAT1 mutations. As measured by CFSE dilution, the healthy, cohabitating ED was enriched for CD8+ T cells that proliferated in response to Candida (Fig. 7A and Supplemental Fig. 4). Likewise, cocultures of T cells and HKCA-presenting monocytes, taken from the ED, produced more IL-6, IFN-γ, and IL-17F compared with healthy controls (Fig. 7B). DLE, derived from this enriched donor, enhanced IL-6 (Fig. 7C) and IL-1β (not shown) from unrelated hDCHKCA, whereas DLE, derived from cells of random healthy donors, failed to induce either. Compared with healthy controls and consistent with the reported negative effects of STAT1 GOF mutations on IL-17A [34], PBMCs from our 2 CMC patients showed increased IL-6 production (Fig. 7D) but reduced IL-17A, IL-17F, and IL-22 upon coculture with autologous hDCHKCA (Fig. 7E). DLE from the ED enhanced IL-1β and IL-6 (Fig. 7D), as well as IL-17F and IL-22 (Fig. 7E), but not IFN-γ (not shown) when added to cocultures of DCHKCA and autologous PBMC from both patients and healthy controls. Interestingly, DLE from the ED failed to rescue the IL-17A defect seen in our CMC patients and in contrast to our murine studies, inhibited IL-17A from healthy volunteers (Fig. 7E). Murine HKCA:DLE also inhibited IL-17A production from human PBMCs cultured with DCHKCA (Fig. 7F), suggesting intrinsic differences between the ability of mouse and human cells to produce IL-17A in response to DLE derived from either species, perhaps reflecting suppression as a result of higher IL-10 production by human cells (Fig. 7G). DLE from both species had similar effects on induction of other cytokines from hDCs, including IL-6 (Fig. 7H) and IL-1β (not shown). Taken together, these results suggest generally conserved DC-stimulating properties of DLE derived from mice and humans.
Figure 7. DLE immune activity is present in humans.
(A and B) Triplicate cultures of monocytes 1:1 with autologous CFSE-loaded CD3+ T cells in the presence of HKCA for 6 d from a Candida exposed donor (ED) who cohabitates with 2 patients with CMC or from random healthy volunteers (HV). On d 6, cells were stained and analyzed for CD3 and CD8 versus CFSE low (CFSElo) and CFSE high cells (A), and concentrations for indicated cytokines in the supernatants were measured (B). (C–H) hDLE was extracted from cellular lysates of PBMCs from ED or different random healthy volunteers. (C) d 3 IL-6 from 1e6 hDCHKCA from unrelated healthy donors (n = 3) and cultured with 1e6 ceq of hDLE from ED or healthy volunteers. (D and E) 4e5 DC from a patient with STAT1 GOF mutation [proband (PB)], her affected daughter (AD), or healthy volunteers were pulsed with HKCA and cultured with 2e6 autologous PBMC and 1e5 ceq of hDLE to assess d 3 IL-1β, IL-6, and IL-10 (D) and IL-17A, IL-17F, and IL-22 (E). (F and G) d 3 IL-17A (F) and IL-10 (G) after 4e5 hDCHKCA were cocultured with 2e6 autologous PBMCs with hDLE or murine HKCA:DLE before (Unsorted) or after bead enrichment for CD8 (CD8 Enr) or depletion (CD8 Dep). (H) IL-6 concentration for 1e6 hDCHKCA was cultured 24 h with 1e5 ceq of hDLE or murine HKCA:DLE. Data shown are representative of 2 (A and B) or 3 or more (C–H) independent experiments using a different healthy volunteer per experiment with triplicate cultures and displayed means ± sem. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, as determined by ANOVA.
DLE binds target antigen
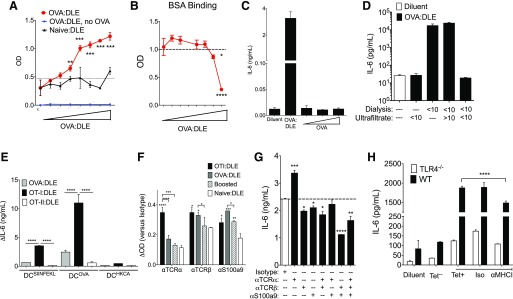
Previous reports suggest that DLE binds its target antigen, as incubation of DLE in antigen-coated plates depleted its immune activity [35, 36]. We confirmed the antigen-binding ability of DLE by finding a dose-dependent increase in OVA binding using OVA:DLE-coated plates but not naïve:DLE-coated plates (Fig. 8A). Wells coated with OVA:DLE but unchallenged with OVA showed no signal, indicating that OVA:DLE did not bind the detection antibody or other reagents (Fig. 8A). Plates coated with OVA:DLE did not enhance binding of BSA (Fig. 8B).
Figure 8. DLE contains S100 peptides and binds target antigen.
(A and B) OVA:DLE or naïve DLE (0 and 1e2–1e8 ceq) was incubated in 96-well immunoplates overnight to replace the capture antibody in a standard sandwich ELISA. OVA or BSA was then added, and signal for bound OVA (A) and BSA (B) is shown versus expected value for the added concentration (dotted, horizontal lines). (C) 1e5 DCOVA were subsequently cultured with diluent; 1e6 OVA:DLE; or 1, 10, or 100 μg e-OVA (no DLE), and IL-6 concentrations were measured after 18–24 h. (D) 1e5 DCOVA cultured with 1e6 ceq of OVA:DLE extracted via ultrafiltration, dialysis, or both. (E) 1e6 DCSIINFEKL, DCOVA, or DCHKCA from C57BL/6 mice were incubated for 24 h with 1e6 ceq of DLE from OVA-immunized mice or unimmunized OT-I or OT-II mice. Change (Δ) in IL-6 compared with diluent condition is shown. (F) Ninety-six-well plates were coated with 1e5 ceq of OT-I, single OVA immunized (OVA:DLE), repeat OVA immunized (Boosted), or naïve:DLE. An ELISA was performed using anti-TCR-α, TCR-β, S100a9, or isotype control as primary antibodies. Absorbance at 405 nm for TCR chains and S100a9 minus the absorbance for isotype control are shown with significance versus naïve:DLE or as indicated. (G) 1e5 DCOVA were incubated with 1e6 ceq of OTI:DLE that had undergone antibody precipitation with antibodies to the TCR α chain (α)TCR-α, TCR-β, and/or S100a9. IL-6 production at 20 h is shown with significance versus the isotype condition (dotted, horizontal line). (H) IL-6 production from 1e6 DCOVA from C57BL/6 wild-type (WT) or TLR4 knockout (TLR4−/−) mice cultured with 5e4 ceq of DLE from CD8+/OVA tetramer-positive (Tet+) or tetramer-negative (Tet−) in the presence of anti-MHC-I or isotype control (Iso). Data shown are representative of 3 or more independent experiments, each using at least 3 mice/group, and displayed as means ± sem. Significance shown versus media control baseline or as indicated. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, as determined by ANOVA.
The active fraction of DLE does not contain antigen contamination
To provide molecular characterization of DLE, we performed peptide sequencing of the crude OVA:DLE from BALB/c mice. Of the >1,000 peptides identified, nearly one-quarter appeared to be derived from S100a9, with S100a912–25 particularly enriched (Supplemental Table 1). S100a9 is an immunologically active, highly post-transcriptionally modified protein with known roles in calcium binding, TLR4 activation, and mucosal inflammation [37, 38]. Of the sequenced peptides lacking matches in protein library databases, VV/ATVSLPR and LSSPATLDSR were the most frequent (Supplemental Table 1). Full-length recombinant S100a9 successfully induced IL-17A and IL-6 from mouse cells, but addition of the recombinant S100a912–25 peptide alone did not (Supplemental Fig. 5A and B). IL-17A and IL-6 (Fig. 5A and B) and footpad swelling (Supplemental Fig. 5C) were enhanced by a combination of S100a912–25, S100a987–100, and VVTVSLPRLSSPATLDSR. However, this activity was not seen when the peptides VVTVSLPR or LSSPATLDSR were used in isolation or when synthetically merged in the inverse orientation. S100-derived peptides were also found in murine OVA:DLE from CD8+ enriched T-cells, murine HKCA:DLE, and hDLE (Supplemental Table 1), suggesting that these are a common feature of DLE across species and antigenic targets. The unmatched sequences, VVTVSLPR and related VVTVVTPR, were only found in the splenocyte- and CD8+ T cell-derived murine OVA:DLE. Their absence from murine HKCA:DLE and hDLE suggested that these sequences may contribute to OVA specificity. Much like our OVA:DLE results (Fig. 8A), the peptide VVTVSLPRLSSPATLDSR, but not the inversely oriented sequence, bound OVA (Supplemental Fig. 5D) but did not enhance BSA binding (not shown), supporting our hypothesis that this peptide contributed to the antigen specificity of OVA:DLE. However, although the reconstituted peptide combination recapitulated each tested aspect of OVA:DLE activity, the cytokine levels induced were less than those seen with OVA:DLE, suggesting that other active components were likely present in the cellular extract beyond the tested peptides.
No peptides from any of the target antigens were found in any samples (Supplemental Table 1), an OVA ELISA was negative for all DLE samples (not shown), and DCs previously saturated with OVA did not produce more IL-6 upon re-exposure to e-OVA antigens (Fig. 8C); each of these findings are inconsistent with the notion that residual antigen contamination in the DLE preparations was the basis for DLE activity. However, in a departure from previous reports of the activity of DLE being found in the <8 kDa fraction [39], further biochemical analysis revealed that IL-6 induction from DCs was discordant between extraction of DLE via ultrafiltration compared with the traditional dialysis method (Fig. 8D). Ultrafiltration of the dialyzed sample revealed that the activity was contained in the >10 kDa fraction, suggesting that the relatively imperfect molecular cutoff of dialysis bags [40] allowed larger, active molecules to leak into the DLE. The DLE preparation was evaluated for cytokine contamination and found to be negative for IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17A, and TNF-α (not shown).
DLE contains TCR chains and S100
We hypothesized that the antigen-binding properties of our DLE preparations were derived from the antigen specificity of the OVA-specific CD8+ T cells in which they were enriched. The antigen specificity of T cells is contained in the hypervariable region of the TCR. To evaluate the potential role for elements of the TCR in the antigen-specific effect of DLE, we derived DLE from C57BL/6-based OT-I and OT-II transgenic mice, which, respectively, display the MHC-I- or MHC-II-restricted, OVA-reactive TCR on all T cells. Despite no direct immunization, DLE extracted from OT-I mice (OT-I:DLE) significantly enhanced IL-6 production from DCOVA (Fig. 8E). DLE extracted from OT-II mice (OT-II:DLE) induced only minimal IL-6 (Fig. 8E). Furthermore, DCSIINFEKL responded to OVA:DLE and OT-I:DLE but not OT-II:DLE (Fig. 8E), consistent with MHC-I-restricted TCR sequences contributing to the antigen specificity of DLE activity.
The partial activity of the OVA-binding peptide and the effectiveness of both the OVA tetramer-positive DLE and OTI:DLE suggested that an interaction between the TCR and MHC-I may also contribute to the observed DLE activity. Consistent with this hypothesis, TCR-α and TCR-β content were both increased in OTI:DLE (Fig. 8F). Likewise, the IL-6 induction by OTI:DLE was significantly reduced by antibody precipitation of TCR-β or S100a9 (Fig. 8G). Precipitation with anti-TCR-α enhanced IL-6 induction, but the combination of anti-TCR-β and anti-S100a9 showed synergistic reduction (Fig. 8G). Similar effects of anti-TCR-β and anti-S100a9 were seen with OVA:DLE derived from actively immunized mice (Supplemental Fig. 5G).
A small but statistically significant reduction of IL-6 production was seen with antibody blockade of MHC-I (Fig. 8H), as well as with use of DCs from the MHC-I-deficient β-2-microglobulin mice (Supplemental Fig. 5H). However, no additional IL-6 reduction was seen in TLR4−/− DCOVA when exposed to anti-MHC-I (Fig. 8H), suggesting combinatorial signaling involving TCR-β interaction with MHC-I, S100a9 interaction with TLR4, and likely other mechanisms that are, as yet, unknown. Taken together, our data suggest that multiple components contribute to DLE activity, including antigen-specific elements derived from the TCR on CD8+ T cells and antigen-independent immune activators, such as S100a9, which operate, in part, through MHC-I and TLR4.
DISCUSSION
We found that the dialysate of immune cell lysates transfers antigen-specific cellular immunity in mouse and human systems. This activity is enriched in CD8+ T cells and acts on antigen-pulsed DCs to induce cytokine responses from memory CD8+ T cells. Fractionation and sequencing of this activity identified a novel peptide sequence capable of binding OVA in BALB/c mice. However, in contrast to prior reports, the OVA:DLE activity was found in the >10 kDa molecular fraction after ultrafiltration. This larger molecular size for DLE activity may alleviate historical detractions for small peptides displaying both antigen specificity and immune activation. This activity was greatly enhanced in OT-I mice that express an OVA-specific TCR, and TCR-β was an active components of OTI:DLE, suggesting that the antigen-specific activator in DLE may be derived from the TCR specificity of the source CD8+ T cell. Of note, the hypervariable region of the OT-I TCR was not found by MS analysis, but this likely represents the trypsin-sensitive sequence of this region [41]. However, whereas immunoprecipitation of TCR-α did not reduce OVA:DLE activity, TCR-α-derived sequences may still contribute to antigen binding and may account for the partial immunologic activity of the identified OVA-binding sequences in OVA:DLE. In contrast, S100-derived sequences were present in the lysate regardless of antigen specificity, species or cellular enrichment, suggesting that they may be a constant, antigen-independent component of DLE activity.
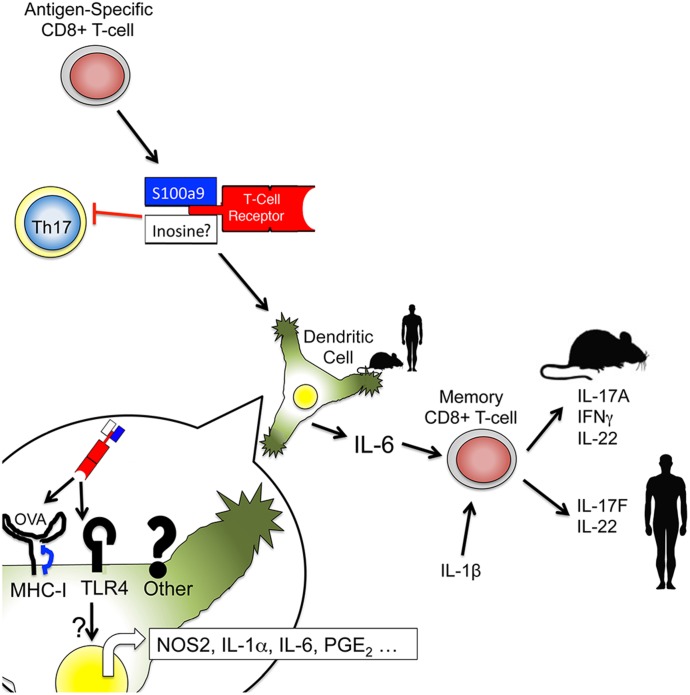
Recently, synthetically produced, soluble TCRs have been used as immunotherapies for the peptide recognition blockade [42] or effector activation when coupled to an immune modulator [43, 44]. Based on our data, we propose a model where DLE activity is conferred by multiple components that may represent a natural form of an activating, soluble TCR therapeutic (Fig. 9). Antigen specificity appears to come from the source CD8+ T cells and depends on sequences likely derived from TCR-β, likely in combination with TCR-α-derived peptide sequences that allow binding of MHC-I-restricted epitopes presented on DCs. Subsequent to DC binding, DC activation may be achieved by the presence of associated immune stimulators, such as S100a9 or the identified S100-derived peptides. Our preliminary data and previous reports suggest that other nonspecific immune stimulators, such as inosine and hypoxanthine, may contribute to this DC activation [45–47]. Secretion of cytokines by the activated DC results in induction of IL-17 and related cytokines from bystander memory CD8+ T cells in a manner that does not require TCR engagement or direct contact with the DC. Our results do not address the validity or mechanistic basis for additional therapeutic claims of DLE that are devoid of antigen targeting [32, 48].
Figure 9. Proposed model of DLE activity.
Antigen-specific CD8+ T cells produce an antigen-specific activator of DCs. An antigen-binding region, likely derived from the TCR-β of the source CD8+ T cell, binds to the cognate antigen presented by DCs. Once bound by the DC, associated DLE molecules, such as S100a9-derived peptides or inosine/hypoxanthine, activate the DC to produce inflammatory cytokines, including IL-6. This cytokine response activates bystander memory CD8+ T cells in a contact- and TCR-independent manner, stimulating the production of IL-17 family cytokines.
As DLE activity is enriched in CD8+ T cells, the antigen specificity of DLE may reflect the polyclonal repertoire of the donor CD8+ T cell population through mechanisms distinct from DTH. This would predict that treatments using DLE from a donor with low numbers of relevant T cells would fail, regardless of the donor ability to mount a positive DTH skin test. In contrast, DLE from an enriched donor would produce more activity but could not necessarily be predicted from DTH positivity. Consistent with this, we found strong immune activation in DLE taken from a healthy donor with high Candida exposure and high anti-Candida reactivity compared with random healthy donors. Whereas the partial dependence of DLE activity on TLR4 signaling could be a result of described TLR4 activation by S100a9 [49, 50], the receptors and molecular details involved in DLE activation of antigen-presenting DCs remain to be fully elucidated.
The observation that >1 immunization resulted in loss of DLE activity (Fig. 3F) may provide insight into discrepancies between earlier DLE studies in guinea pigs. Studies using single immunization protocols found antigen-specific DLE activity [51], whereas experiments relying on DTH responses and repeated immunizations before lysate extraction showed either no effect or only nonantigen-specific effects [52]. The mechanistic basis for loss of DLE activity with repeated immunization is not clear but may be related to alterations in the content of costimulators, such as S100a9. Furthermore, the recent distinction between effector and central memory cells may also contribute, with the former being preferentially induced by prolonged, low-grade antigen exposure and the latter being favored by repeated intermittent exposure [53]. It is possible that within the highly unique memory CD8+ T cell compartment [54], DLE activity is segregated further to effector rather than central memory cells. Limitation in the ability to isolate adequate numbers of each population has precluded us from directly testing this hypothesis. Interestingly, effector memory CD8+ T cells are enriched in the colostrum of both humans [55] and cows [56], and PPD reactivity has been suggested to be transferable through breastfeeding [57], raising the intriguing possibility that DLE activity represents a mechanism for transfer of maternal cellular immunity to the immunologically naïve newborn.
The potential to transfer passively cellular immunity for treatment of infectious and malignant diseases will depend on understanding and harnessing its antigen-specific properties. The capacity of CD8+ T cells to harbor this activity suggests that they may play a physiologic role in TCR-independent recruitment and/or activation of bystander CD8+ T cells via antigen-specific activation of DCs during antigen exposure. However, the physiologic relevance of Tc17 cells is not yet clear beyond their potential contribution to rheumatologic pathology [27]. Numerous reports have shown that Tc17 cells in vitro rapidly convert to coproducers of IFN-γ when transferred in vivo or exposed to IL-12 or IL-23 [27, 28, 58, 59]. Given the partial IFN-γ dependence seen in our footpad experiments, it seems likely that the differences in the physiology of Tc17 cells in vivo versus in vitro may also impact responses to DLE. The mechanism by which DLE activity is limited to CD8+ T cells will be an interesting area of future research but may be a result of reported differences in the content and phosphorylation of the TCR-associated intracellular tails and lipid rafts between CD8+ and CD4+ T cells [60]. Overall, our work makes it clear that lysates from CD8+ T cells can transfer passive cellular immunity, setting the stage for future work to elucidate more fully the structure, physiologic role, and therapeutic potential of DLE.
AUTHORSHIP
I.A.M. designed, conducted, or assisted on all experiments and wrote the manuscript. M.Z. and G.N. performed the protein chemistry experiments. L.R.O. performed amino acid sequencing. D.S. and J.D.R. provided technical assistance on select experiments. T.J.B. and M.S.L. performed experiments on mouse models in candidiasis. T.G.M. and P.J.G. performed all RNA-seq experiments. C.H.K. provided consultation on experimental design. S.M.H. oversaw the clinical care of the patients with STAT1 GOF mutations. S.K.D. oversaw the project and helped design and analyze the experiments. All authors critically read the manuscript.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of NIAID and the U.S. National Institutes of Health. The authors thank the patients, their families, and healthy participants for their assistance in this project. The authors also thank the NIAID Building 33 and 14BS veterinarians, animal care, and breeder technicians for their assistance on animal experiments; Drs. Bishop Hague and Elina Stregevsky for help with cell sorting; Dr. Francisco Otaizo-Carrasquero for help performing RNA-seq; Mark Kieh and Elim Cho for technical assistance; and Dawn Shaw, Towanda Carroll, Bevin Feutier, Emily Franks, Yuka Kanno, Kimberly Hilsen, Raynaldo Martin (NIAID), and Anthony St. Leger (NEI) for administrative assistance, as well as Mr. and Mrs. Topolino (NIAID) for their cooperation and sacrifice during the course of this project.
Glossary
- C3H
C3H/HeJ
- CD62L
cluster of differentiation 62 ligand
- ceq
cellular equivalents
- CMC
chromic mucocutaneous Candiasis
- DC
dendritic cell
- DCX
dendritic cell pulsed with X antigen
- dko
double-knockout
- DLE
dialyzed leukocyte extract
- DTH
delayed-type hypersensitivity
- e-OVA
endotoxin-free OVA
- ED
exposed donor
- FDR
false discovery rate
- GOF
gain of function
- hDC
human dendritic cell
- hDLE
human dialyzed leukocyte extract
- HIES
hyper-IgE syndrome
- HKCA
heat-killed Candida albicans
- IRB
Institutional Review Board
- LTQ
linear trap quadropole
- MHC-I /II
MHC class I/II
- moDC
monocyte-derived dendritic cell
- MRSA
methicillin-resistant Staphylococcus aureus
- MS
mass spectrometry
- NEI
National Eye Institute
- NIAID
National Institute of Allergy and Infectious Diseases
- PPD
purified protein derivative
- RNA-seq
RNA sequencing
- SIINFEKL
MHC class I-restricted OVA peptide
- Stat3HIES
hyper-IgE syndrome that expresses a dysfunctional Stat3
- Tc17
CD8+, IL-17-producing T cell(s)
- Th17
CD4+, IL-17-producing T cell(s)
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors have no conflicts of interest.
REFERENCES
- 1.Landsteiner K., Chase M. W. (1942) Experiments on transfer of cutaneous sensitivity to simple compounds. Exp. Biol. Med. (Maywood) 49, 688–690. [Google Scholar]
- 2.Lawrence H. S. (1955) The transfer in humans of delayed skin sensitivity to streptococcal M substance and to tuberculin with disrupted leucocytes. J. Clin. Invest. 34, 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence H. S., Pappenheimer A. M. Jr. (1956) Transfer of delayed hypersensitivity to diphtheria toxin in man. J. Exp. Med. 104, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger D. R., Vandenbark A. A., Daves D., Anderson W. A. Jr.,Vetto R. M., Finke P. (1976) Human transfer factor: fractionation and biologic activity. J. Immunol. 117, 789–796. [PubMed] [Google Scholar]
- 5.Sargent I. L., Salaman M. R. (1980) Effects of human transfer factor on the migration of guinea-pig macrophages: is there an antigen-specific activity? Immunology 41, 227–235. [PMC free article] [PubMed] [Google Scholar]
- 6.Salaman M. R. (1978) An investigation into the antigen-specificity of transfer factor in its stimulatory action on lymphocyte transformation. Immunology 35, 247–256. [PMC free article] [PubMed] [Google Scholar]
- 7.Steele R. W., Myers M. G., Vincent M. M. (1980) Transfer factor for the prevention of varicella-zoster infection in childhood leukemia. N. Engl. J. Med. 303, 355–359. [DOI] [PubMed] [Google Scholar]
- 8.Arala-Chaves M. P., Proença R., De Sousa M. (1974) Transfer factor therapy in a case of complex immunodeficiency. Cell. Immunol. 10, 371–379. [DOI] [PubMed] [Google Scholar]
- 9.Gallin J. I., Kirkpatrick C. H. (1974) Chemotactic activity in dialyzable transfer factor. Proc. Natl. Acad. Sci. USA 71, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oettgen H. F., Old L. J., Farrow J. H., Valentine F. T., Lawrence H. S., Thomas L. (1974) Effects of dialyzable transfer factor in patients with breast cancer. Proc. Natl. Acad. Sci. USA 71, 2319–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng R. P., Moran C. J., Alexopoulos C. G., Bellingham A. J. (1975) Transfer factor in Hodgkin's disease. Lancet 2, 901–903. [DOI] [PubMed] [Google Scholar]
- 12.Fudenberg H. H. (1976) Dialyzable transfer factor in the treatment of human osteosarcoma: an analytic review. Ann. N. Y. Acad. Sci. 277, 545–557. [DOI] [PubMed] [Google Scholar]
- 13.Sawyer M., Willadsen C. H., Osburn B. I., McGuire T. C. (1977) Passive transfer of colostral immunoglobulins from ewe to lamb and its influence on neonatal lamb mortality. J. Am. Vet. Med. Assoc. 171, 1255–1259. [PubMed] [Google Scholar]
- 14.Franco-Molina M. A., Mendoza-Gamboa E., Castillo-Tello P., Isaza-Brando C. E., Garcia M. E., Castillo-León L., Tamez-Guerra R. S., Rodríguez-Padilla C. (2007) Bovine dialyzable leukocyte extract modulates cytokines and nitric oxide production in lipopolysaccharide-stimulated human blood cells. Cytotherapy 9, 379–385. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y., Dorf M. E. (2001) Delayed-type hypersensitivity. Curr. Protoc. Immunol. Chapter 4, Unit 4.5. [DOI] [PubMed] [Google Scholar]
- 16.Gaidamakova E. K., Myles I. A., McDaniel D. P., Fowler C. J., Valdez P. A., Naik S., Gayen M., Gupta P., Sharma A., Glass P. J., Maheshwari R. K., Datta S. K., Daly M. J. (2012) Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radio-protective Mn2+-peptide complex from Deinococcus. Cell Host Microbe 12, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Neill D. W., Bhardwaj N. (2005) Differentiation of peripheral blood monocytes into dendritic cells. Curr. Protoc. Immunol. Chapter 22, Unit 22F.4. [DOI] [PubMed] [Google Scholar]
- 18.Nair S., Archer G. E., Tedder T. F. (2012) Isolation and generation of human dendritic cells. Curr. Protoc. Immunol. Chapter 7, Unit 7.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acosta-Rodriguez E. V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8, 639–646. [DOI] [PubMed] [Google Scholar]
- 20.Break T. J., Jaeger M., Solis N. V., Filler S. G., Rodriguez C. A., Lim J. K., Lee C. C., Sobel J. D., Netea M. G., Lionakis M. S. (2015) CX3CR1 is dispensable for control of mucosal Candida albicans infections in mice and humans. Infect. Immun. 83, 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myles I. A., Fontecilla N. M., Valdez P. A., Vithayathil P. J., Naik S., Belkaid Y., Ouyang W., Datta S. K. (2013) Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat. Immunol. 14, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkpatrick C. H. (2000) Transfer factors: identification of conserved sequences in transfer factor molecules. Mol. Med. 6, 332–341. [PMC free article] [PubMed] [Google Scholar]
- 23.Naisbitt D. J., Nattrass R. G., Ogese M. O. (2014) In vitro diagnosis of delayed-type drug hypersensitivity: mechanistic aspects and unmet needs. Immunol. Allergy Clin. North Am. 34, 691–705, x. [DOI] [PubMed] [Google Scholar]
- 24.Fong T. A., Mosmann T. R. (1989) The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J. Immunol. 143, 2887–2893. [PubMed] [Google Scholar]
- 25.Steward-Tharp S. M., Laurence A., Kanno Y., Kotlyar A., Villarino A. V., Sciume G., Kuchen S., Resch W., Wohlfert E. A., Jiang K., Hirahara K., Vahedi G., Sun H. W., Feigenbaum L., Milner J. D., Holland S. M., Casellas R., Powrie F., O’Shea J. J. (2014) A mouse model of HIES reveals pro- and anti-inflammatory functions of STAT3. Blood 123, 2978–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdez P. A., Vithayathil P. J., Janelsins B. M., Shaffer A. L., Williamson P. R., Datta S. K. (2012) Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity 36, 668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang Y., Pan H. F., Ye D. Q. (2015) Tc17 cells in immunity and systemic autoimmunity. Int. Rev. Immunol. 34, 318–331. [DOI] [PubMed] [Google Scholar]
- 28.Yen H. R., Harris T. J., Wada S., Grosso J. F., Getnet D., Goldberg M. V., Liang K. L., Bruno T. C., Pyle K. J., Chan S. L., Anders R. A., Trimble C. L., Adler A. J., Lin T. Y., Pardoll D. M., Huang C. T., Drake C. G. (2009) Tc17 CD8 T cells: functional plasticity and subset diversity. J. Immunol. 183, 7161–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappu R., Rutz S., Ouyang W. (2012) Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 33, 343–349. [DOI] [PubMed] [Google Scholar]
- 30.Park C. O., Kupper T. S. (2015) The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J., Kong C., Wang X., Shao J., Feng L., Zhang Z. (2015) Preparation and identification of transfer factor specific to Staphylococcus aureus in vitro. Biotechnol. Appl. Biochem. 62, 112–120. [DOI] [PubMed] [Google Scholar]
- 32.Viza D., Fudenberg H. H., Palareti A., Ablashi D., De Vinci C., Pizza G. (2013) Transfer factor: an overlooked potential for the prevention and treatment of infectious diseases. Folia Biol. (Praha) 59, 53–67. [PubMed] [Google Scholar]
- 33.Conti H. R., Gaffen S. L. (2015) IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J. Immunol. 195, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazaki Y., Yamada M., Kawai T., Morio T., Onodera M., Ueki M., Watanabe N., Takada H., Takezaki S., Chida N., Kobayashi I., Ariga T. (2014) Two novel gain-of-function mutations of STAT1 responsible for chronic mucocutaneous candidiasis disease: impaired production of IL-17A and IL-22, and the presence of anti-IL-17F autoantibody. J. Immunol. 193, 4880–4887. [DOI] [PubMed] [Google Scholar]
- 35.Pannetier C., Cochet M., Darche S., Casrouge A., Zoller M., Kourilsky P (1993) The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc. Natl. Acad. Sci. USA 90, 4319–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozzo S. J., Kirkpatrick C. H. (1992) Purification of transfer factors. Mol. Immunol. 29, 167–182. [DOI] [PubMed] [Google Scholar]
- 37.Markowitz J., Carson W. E. III (2013) Review of S100A9 biology and its role in cancer. Biochim. Biophys. Acta 1835, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loser K., Vogl T., Voskort M., Lueken A., Kupas V., Nacken W., Klenner L., Kuhn A., Foell D., Sorokin L., Luger T. A., Roth J., Beissert S. (2010) The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 16, 713–717. [DOI] [PubMed] [Google Scholar]
- 39.Kirkpatrick C. H. (1996) Activities and characteristics of transfer factors. Biotherapy 9, 13–16. [DOI] [PubMed] [Google Scholar]
- 40.Leypoldt J. K., Hoff C. M., Piscopo D., Carr S. N., Svatek J. M., Holmes C. J. (2013) Ultrafiltration characteristics of glucose polymers with low polydispersity. Perit. Dial. Int. 33, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. (1994) T Cell receptor antagonist peptides induce positive selection. Cell 76, 17–27. [DOI] [PubMed] [Google Scholar]
- 42.Molloy P. E., Sewell A. K., Jakobsen B. K. (2005) Soluble T cell receptors: novel immunotherapies. Curr. Opin. Pharmacol. 5, 438–443. [DOI] [PubMed] [Google Scholar]
- 43.Boulter J. M., Jakobsen B. K. (2005) Stable, soluble, high-affinity, engineered T cell receptors: novel antibody-like proteins for specific targeting of peptide antigens. Clin. Exp. Immunol. 142, 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosquera L. A., Card K. F., Price-Schiavi S. A., Belmont H. J., Liu B., Builes J., Zhu X., Chavaillaz P. A., Lee H. I., Jiao J. A., Francis J. L., Amirkhosravi A., Wong R. L., Wong H. C. (2005) In vitro and in vivo characterization of a novel antibody-like single-chain TCR human IgG1 fusion protein. J. Immunol. 174, 4381–4388. [DOI] [PubMed] [Google Scholar]
- 45.Tomar R. H., Knight R., Stern M. (1976) Transfer factor: hypoxanthine is a major component of a fraction with in vivo activity. J. Allergy Clin. Immunol. 58, 190–197. [DOI] [PubMed] [Google Scholar]
- 46.Batal I., Azzi J., Mounayar M., Abdoli R., Moore R., Lee J. Y., Rosetti F., Wang C., Fiorina P., Sackstein R., Ichimura T., Abdi R. (2014) The mechanisms of up-regulation of dendritic cell activity by oxidative stress. J. Leukoc. Biol. 96, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan L., Campbell D. H. (1971) A unique low molecular weight antigen fragment associated with oligoribonucleopeptide in lymph nodes and spleen. Immunochemistry 8, 185–199. [DOI] [PubMed] [Google Scholar]
- 48.Vetto R. M., Burger D. R., Nolte J. E., Vandenbark A. A., Baker H. W. (1976) Transfer factor therapy in patients with cancer. Cancer 37, 90–97. [DOI] [PubMed] [Google Scholar]
- 49.Gao H., Zhang X., Zheng Y., Peng L., Hou J., Meng H. (2015) S100A9-induced release of interleukin (IL)-6 and IL-8 through toll-like receptor 4 (TLR4) in human periodontal ligament cells. Mol. Immunol. 67 (2 Pt B), 223–232. [DOI] [PubMed] [Google Scholar]
- 50.Chen B., Miller A. L., Rebelatto M., Brewah Y., Rowe D. C., Clarke L., Czapiga M., Rosenthal K., Imamichi T., Chen Y., Chang C. S., Chowdhury P. S., Naiman B., Wang Y., Yang D., Humbles A. A., Herbst R., Sims G. P. (2015) S100A9 induced inflammatory responses are mediated by distinct damage associated molecular patterns (DAMP) receptors in vitro and in vivo. PLoS One 10, e0115828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turk J. L., Asherson G. L. (1962) Attempts to transfer contact sensitivity passively with subcellular fractions in the guinea pig. A study of the specificity of such reactions. Int. Arch. Allergy Appl. Immunol. 21, 321–325. [DOI] [PubMed] [Google Scholar]
- 52.Bloom B. R., Chase M. W. (1967) Transfer of delayed-type hypersensitivity. A critical review and experimental study in the guinea pig. Prog. Allergy 10, 151–255. [PubMed] [Google Scholar]
- 53.Pepper M., Jenkins M. K. (2011) Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 12, 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willinger T., Freeman T., Hasegawa H., McMichael A. J., Callan M. F. (2005) Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J. Immunol. 175, 5895–5903. [DOI] [PubMed] [Google Scholar]
- 55.Wirt D. P., Adkins L. T., Palkowetz K. H., Schmalstieg F. C., Goldman A. S. (1992) Activated and memory T lymphocytes in human milk. Cytometry 13, 282–290. [DOI] [PubMed] [Google Scholar]
- 56.Yang T. J., Ayoub I. A., Rewinski M. J. (1997) Lactation stage-dependent changes of lymphocyte subpopulations in mammary secretions: inversion of CD4+/CD8+ T cell ratios at parturition. Am. J. Reprod. Immunol. 37, 378–383. [DOI] [PubMed] [Google Scholar]
- 57.Schlesinger J. J., Covelli H. D. (1977) Evidence for transmission of lymphocyte responses to tuberculin by breast-feeding. Lancet 2, 529–532. [DOI] [PubMed] [Google Scholar]
- 58.Satoh T., Tajima M., Wakita D., Kitamura H., Nishimura T. (2012) The development of IL-17/IFN-γ-double producing CTLs from Tc17 cells is driven by epigenetic suppression of Socs3 gene promoter. Eur. J. Immunol. 42, 2329–2342. [DOI] [PubMed] [Google Scholar]
- 59.Kondo T., Takata H., Matsuki F., Takiguchi M. (2009) Cutting edge: phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J. Immunol. 182, 1794–1798. [DOI] [PubMed] [Google Scholar]
- 60.Kannan A., Huang W., Huang F., August A. (2012) Signal transduction via the T cell antigen receptor in naïve and effector/memory T cells. Int. J. Biochem. Cell Biol. 44, 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]