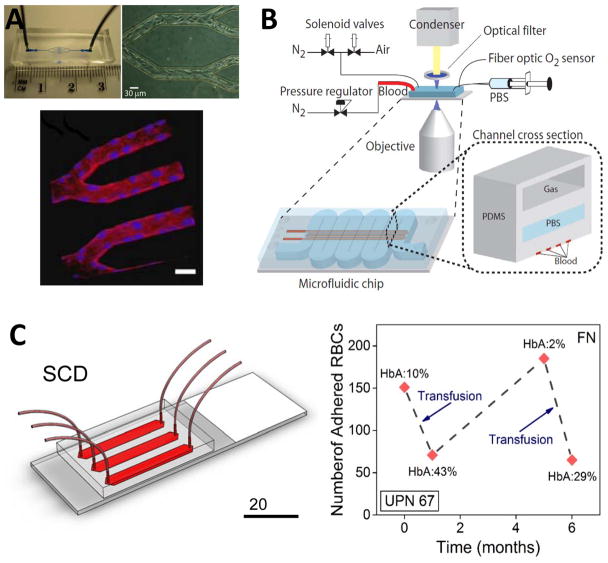
Figure 3. Emerging POC technologies for SCD monitoring.
(A) An endothelialized microfluidic platform to model microvascular occlusion in vitro247. Brightfield microscopy shows the immobilized endothelial cells 48 hours after the cells are seeded in the microfluidic device. The cultured cells round up the rectangular cross-section mimicking in vivo blood vessel geometry and size scale. Immobilized endothelial cells round up the rectangular cross-section mimicking in vivo blood vessel geometry and size scale. The endothelialized microfluidic platform can recapitulate altered blood flow and occlusive events in physiologically relevant flow conditions. The influence of hydroxyurea treatment of sickle cell patients on cell adhesion and subsequent microvascular occlusion is also observed using fluorescent microscopy. Reproduced with permission from [247] (B) A microfluidic platform to probe blood rheology of SCD patient blood samples in physiologically relevant flow and oxygen tension conditions243. Blood flow conductance can be measured in microfluidic channels at the bottom layer, whereas the channel at the top layer are filled with N2 to deoxygenate the blood through gas diffusion across the separating PDMS membrane. Reproduced with permission from [243] (C) SCD Biochip as a functional RBC adhesion assay to monitor SCD34. Adhesion of RBCs to endothelium and sub-endothelium associated proteins in physiologically relevant size scale and flow conditions are analyzed and associated with clinical course of SCD patients. SCD Biochip provides rapid, fully enclosed, standardized, and pre-processing-free analysis of RBC adhesion in whole SCD patient blood samples, enabling longitudinal studies.