Fig. 7.
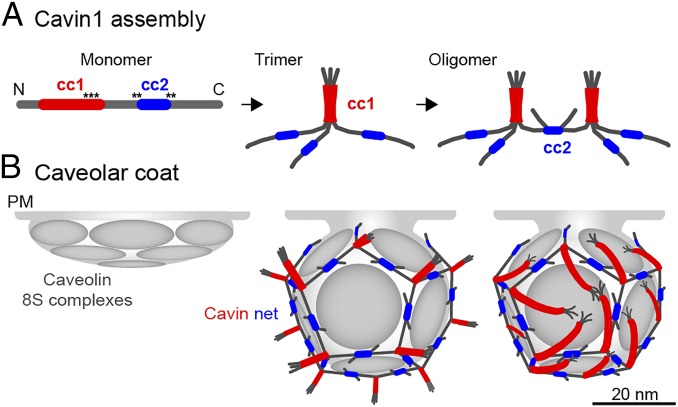
A model for Cavin1 complex assembly and caveolar coat architecture. In our speculative model, Cavin1 monomers form trimers and then assemble into larger, interconnected oligomers (A) to form a dodecahedron around the caveolar membrane (B). Stoichiometry and estimated particle size of the Cavin1 60S complex suggest that caveolae contain one such complex each. Trimers formed by cc1 interactions are localized to the 20 vertices and are connected through dimeric cc2 interactions along the edges. The cc1 domains (red) form a parallel trimeric coiled-coil generating a 17-nm-long, relatively stiff rod-like structure with a PIP2 binding region about 2–3 nm from the C-terminal end of the coil (black asterisks). There is limited information for cc2 (blue), and we present dimeric, antiparallel interactions in our model. Contacts with the membrane are mediated by PS-binding through residues that flank and possibly include cc2 and by PIP2 binding to cc1 (black asterisks). In addition, there possibly are weak interactions with CAV1. The trimers are likely positioned parallel to the surface of the caveolar membrane and could generate the striations seen on the surface of caveolae in previous EM studies. The model leaves the sequences N-terminal to cc1 (amino acids 1–57) and C-terminal to cc2 (amino acids 285–392) free; they are external to the coat. Because they contain many potential phosphorylation sites, they likely are involved in the interaction with additional proteins. Cavin2 and Cavin3 (not depicted) may induce breaks in the polyhedron because they do not contain cc2 and thereby could allow the formation of the neck of caveolae. The 12 faces of the dodecahedron are likely to be occupied by disc-shaped caveolin oligomers that float in the underlying membrane and thus can interact with each other, lipids, and the appressed cavin coat. It is likely that the caveolin domain requires the cavin coat to induce and maintain the curvature and hence the invaginated shape of caveolae.