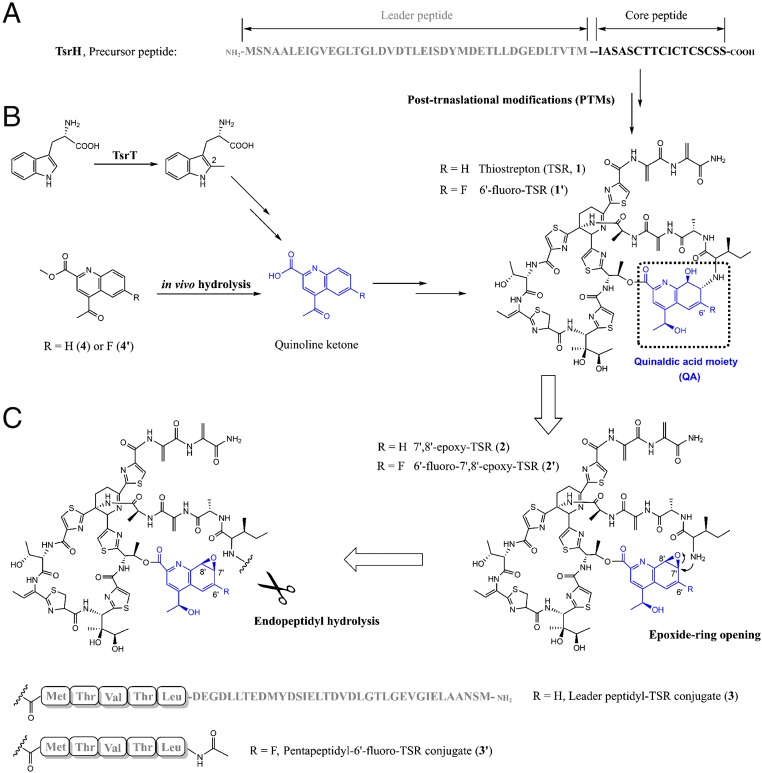
Fig. 1.
The biosynthesis of TSR through PTMs of a genetically encoded precursor peptide. (A) TsrH, the precursor peptide of TSR that is composed of a leader peptide (gray) and a core peptide (black). (B) The (engineered) pathway of the (6′-fluorinated) QA moiety (blue) and its incorporation with the core peptide-derived thiopeptide framework to produce TSR (1) or 6′-fluoro-TSR (1′). (C) Retro-biosynthetic analysis of the construction of the side-ring system of 1 or 1′, showing here the necessity of the dual activity of TsrI for both C-N bond cleavage (endopeptidyl hydrolysis) and formation (epoxide ring opening).