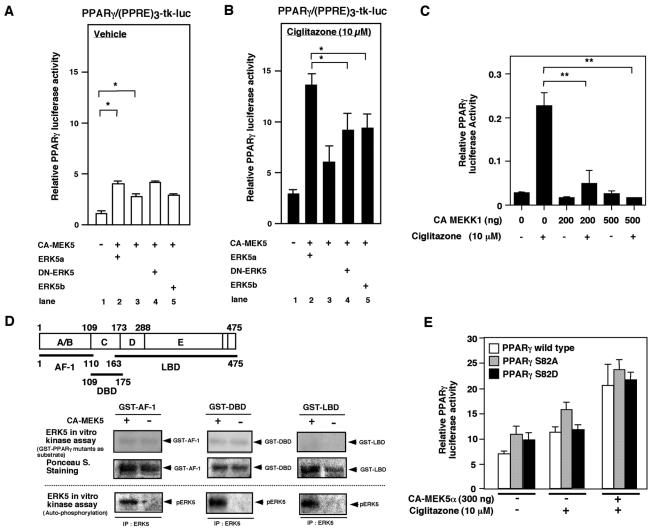
FIG. 1.
MEK5-ERK5 activation increases PPARγ1-mediated transactivation of the (PPRE)3-tk-luciferase reporter construct in HUVECs, which is independent of PPARγ1 S82 phosphorylation. (A and B) MEK5-ERK5 activation induced PPARγ1 transcriptional activity, but DN-ERK5 did not inhibit CA-MEK5α-mediated PPARγ activity. PPARγ1 transcriptional activity was measured by transfection of full-length PPARγ1 and the (PPRE)3-tk-luciferase reporter construct in HUVECs. PPARγ-mediated transactivation was determined with the transfection of wild-type ERK5 (ERK5a, lane2), empty vector (lane 3), or ERK5 mutants (DN-ERK5 [lane 4] or ERK5b [lane5]) with vehicle (A) or 10 μM ciglitazone (B). Results are the mean ± SD of three to six independent experiments. Luciferase activity of the (PPRE)3-tk-luc construct with CA-MEK5α and ERK5a in the absence of transfected PPARγ at ciglitazone concentrations of 0 and 10 μM were 0.8 ± 0.2 and 1.1 ± 0.2 (relative PPARγ luciferase activity), respectively. Luciferase activity of the TK promoter alone with CA-MEK5α and ERK5a was below 0.1 relative PPARγ luciferase activity. (C) Activation of MEKK1 inhibited PPARγ activation. CA-MEKK1, as indicated, was transfected in Cos7 cells, and pcDNA3.1 vector was used to provide equal amounts of transfected DNA. Results are the mean ± SD of three independent experiments. (D) ERK5 did not phosphorylate PPARγ1 in an in vitro kinase assay. CHO cells were transfected with vector or CA-MEK5α, and ERK5 was immunoprecipitated with ERK5 antibody. An immune complex kinase assay was then performed with GST or GST-PPARγ1 mutants (GST-PPARγ1-AF-1, GST-PPARγ1-DBD, and GST-PPARγ1-LBD). (E) CA-MEK5α- and/or ciglitazone-induced full-length PPARγ1 wild type or mutants (PPARγ1S82A or PPARγ1S82D) mediated transactivation of the (PPRE)3-tk-luciferase reporter construct in HUVECs. Results are the mean ± SD of three independent experiments.