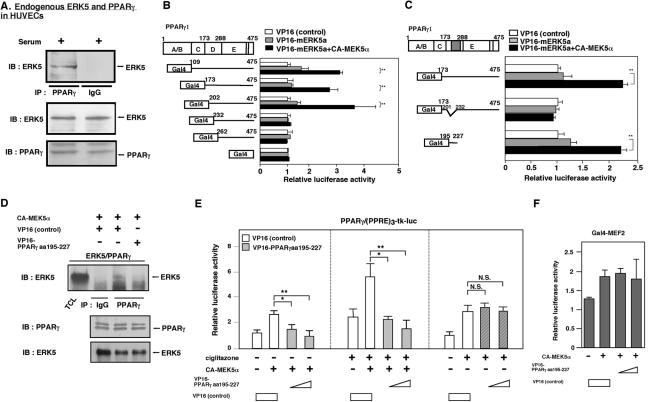
FIG. 2.
Endogenous ERK5 associates with endogenous PPARγ at the hinge-helix 1 region of PPARγ1, and the hinge-helix 1 region of the PPARγ1 fragment inhibited the ERK5-PPARγ interaction and CA-MEK5α-mediated PPARγ transcriptional activity. (A) HUVECs were stimulated with 10% serum for 30 min, whole-cell extract was immunoprecipitated with anti-PPARγ antibody or an equal amount of rabbit IgG, and Western blot analysis was performed with anti-ERK5 antibody (top). No difference in the amount of ERK5 (middle) or PPARγ (bottom) was observed in samples by Western blot analysis with anti-ERK5 (middle) or anti-PPARγ (bottom) antibody. (B and C) Association of activated ERK5a with PPARγ1 hinge-helix 1 was tested in a mammalian two-hybrid assay. The activation domain VP16 was fused to wild-type ERK5a and the PPARγ1 deletion mutants. Luciferase activity was normalized relative to the mean luciferase activity of the empty VP16 transfection (white bar; set as 1-fold). Constructs fused to the Gal4 binding domain were cotransfected with the Gal4-responsive luciferase reporter pG5-luc with or without cotransfection of CA-MEK5α in Cos7 cells for 40 h. The total transfected DNA amount was normalized with empty VP16 vector. Results are the mean ± SD of three independent experiments (B). (C) Association of activated ERK5a with PPARγ1 hinge-helix 1 was tested with PPARγ1 hinge-helix 1 truncated deletion mutant (PPARγ1 Δaa202-231) or small fragment of PPARγ1 (aa195-227) with or without CA-MEK5α or VP16-ERK5a, in a mammalian two-hybrid assay. The total transfected DNA amount was normalized with empty VP16 vector. (D) The VP16-PPARγ1(aa 195-227) fragment inhibited coimmunoprecipitation of ERK5 with PPARγ (top). No difference in the amount of PPARγ (middle) or ERK5 (bottom) was observed in samples by Western blot analysis with anti-PPARγ (middle) or anti-ERK5 (bottom) antibody. (E) Cells were transfected with plasmids expressing VP16 or the VP16-PPARγ1(aa 195-227) fragment and 1 μg of (PPRE)3-tk-luciferase, 0.5 μg of pSG5-PPARγ, and vector to provide equal amounts of transfected DNA, as described for Fig. 1. After 16 h of stimulation with or without ciglitazone, luciferase PPARγ1 transcriptional activity was assayed as described in the legend for Fig. 1. The total transfected DNA amount was normalized with empty VP16 vector. Results are the mean ± SD of three to six independent experiments. (F) Cotransfection of plasmid expressing the VP16-PPARγ1(aa 195-227) fragment did not inhibit CA-MEK5α-induced PPARγ1 activation in HUVECs. The total transfected DNA amount was normalized with empty VP16 vector.