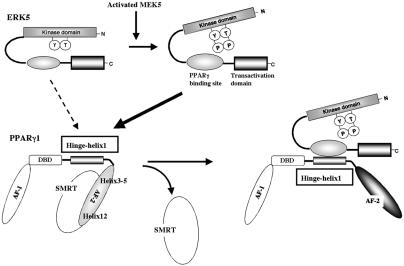
FIG. 9.
Model of the ERK5a-PPARγ1 interaction activating PPARγ1 activity. The position of H12 is regulated by a ligand. In the ligand binding receptor, H12 folds back to form part of the coactivator binding surface. By contrast, H12 inhibits corepressor binding to PPARγ and other nuclear receptors (29). The corepressor interaction surface requires H3, H4, and H5, thereby overlapping the coactivator interaction surface (14). In the present study we found a critical role for the hinge-helix 1 domain in regulating PPARγ1 transcriptional activity. The inactive NH2-terminal kinase domain of ERK5a partially inhibits the association of PPARγ1 on COOH-terminal ERK5 and also inhibits its transcriptional activity. Following activation, the inhibitory effect of NH2-terminal ERK5 decreases, and the middle region of ERK5a fully interacts with the hinge-helix 1 region of PPARγ1. The association of ERK5a with the hinge-helix 1 region of PPARγ1 releases corepressor SMRT and induces full activation of PPARγ1.