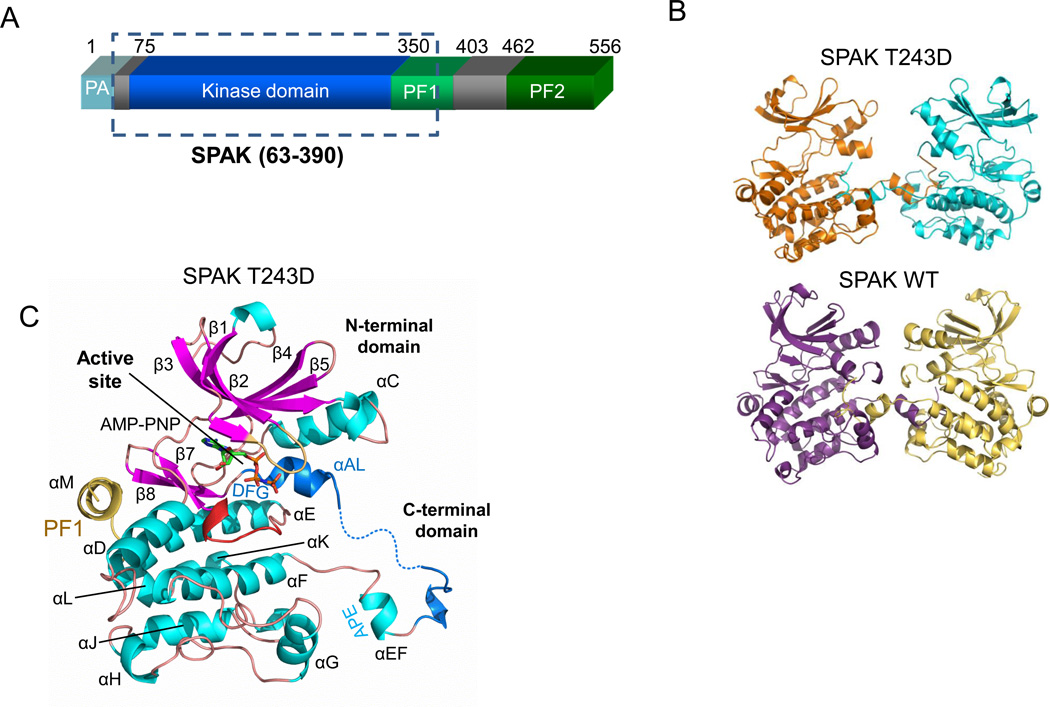
Figure 1. Domain organization and structure of SPAK WT and T243D.
(A) Domain organization of mouse SPAK. The kinase domain (residue 75–350) is shown in blue, the PF1 domain (residues 350–403) in green, and the PF2 domain (residue 462–556) in darker green. The SPAK T243D fragment crystallized is boxed. (B) Activation loop domain-swapped dimers of SPAK T243D and SPAK WT. The two SPAK T243D dimer subunits are colored orange and blue, and the two SPAK WT subunits are colored purple and yellow. (C) Ribbon diagram of a SPAK T243D subunit (or monomer) as observed in the domain-swapped dimer. α-helices-cyan; β-strands-magenta; glycine-rich loop- brown; catalytic loop- red; activation loop- blue; PF1 domain- yellow. AMP-PNP is shown in a stick representation.