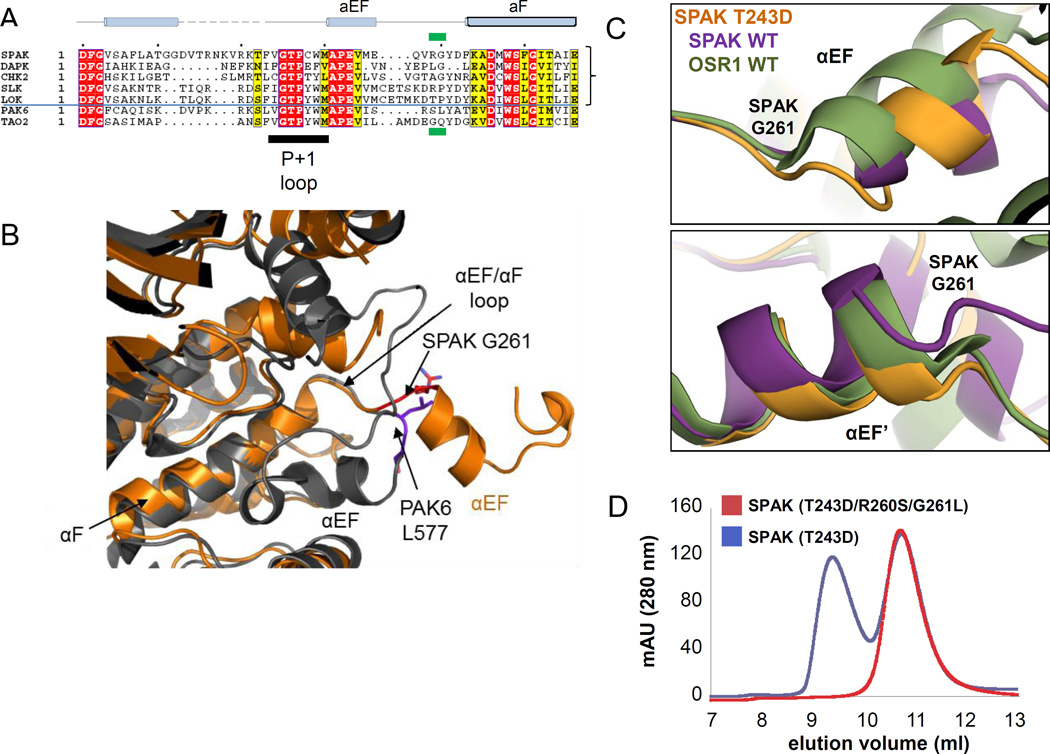
Figure 3. Identification and mutation of the domain-swap hinge.
(A) Multiple sequence alignment of representative sequences of domain swapped kinases SPAK, DAPK3, CHK2, SLK, LOK, compared to the non-swapped kinases PAK6 and TAO2. The position corresponding to G261 in SPAK is boxed in green. (B) Superposition of SPAK T243D and the non-swapped kinase PAK6 (PDB 2C30) reveals the difference in the αEF/αF loop. SPAK is colored in orange and PAK6 is in gray. The switch point R260/G261 in SPAK and S576/L577 in PAK6 are represented in sticks and colored in red and purple, respectively. (C) View near αEF and αEF’ helices using chain A structural alignments of SPAK WT, SPAK T243D, and OSR1 WT dimers. Positional differences begin to occur near G261 and G261' (G203 in OSR1). (D) Gel filtration profile of SPAK T243D and SPAK (T243D/R260S/G261L).