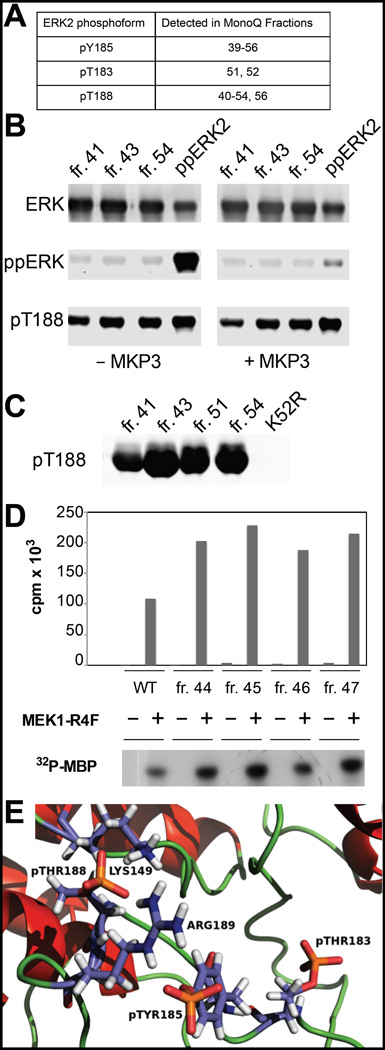
Figure 2.
Phosphorylated T188 (pT188) is present in bacterial preparations of ERK2. (A) Summary of MS analysis of fractions from a MonoQ purification of ERK2. (B) Selected fractions were immunoblotted with antibodies that recognize ERK2 regardless of its phosphorylation state, activated ERK2 (ppERK2), and pT188 selectively15. MAP kinase phosphatase 3 (MKP3) is able to dephosphorylate pY185 and pT183 but not pT188. (C) Immunoblot of MonoQ fractions of purified wild type ERK2 and a preparation of a kinase-dead ERK2 (K52R) with a pT188-selective antibody. (D) MEK1R4F recognizes and phosphorylates eluted fractions containing pT188, increasing ERK2 kinase activity toward MBP. (E) Model of the triply phosphorylated ERK2 activation loop.