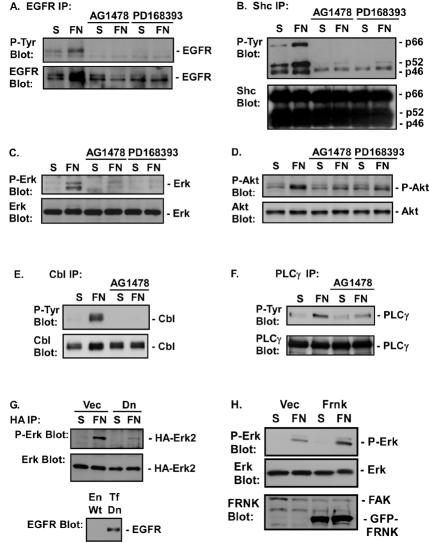
FIG. 2.
EGFR is required for FN-induced signaling events. Cos7 cells were placed in suspension (S), either left untreated or treated with 1 μM AG1478 or 0.5 μM PD168393, and plated on FN for 30 min. (A through F) EGFR (A), Shc (B), Erk (C), Akt (D), Cbl (E), and PLCγ (F) phosphorylation was analyzed by immunoblotting of EGFR, Shc, PLCγ, or Cbl immunoprecipitates with an anti-phosphotyrosine monoclonal antibody (P-Tyr Blot) or by immunoblotting of total-cell extracts with a phosphospecific antibody against Erk (P-Erk Blot) or Akt (P-Akt Blot). Total levels of EGFR, Shc, Erk, Akt, PLCγ, or Cbl in the immunoprecipitates or cell lysates were analyzed by immunoblotting with their respective antibodies (EGFR Blot, Shc Blot, Erk Blot, Akt Blot, PLCγ Blot, and Cbl Blot). (G) Cos7 cells were transfected with 0.2 μg of pSLV-HA-Erk2 and 1 μg of pDEST12.2-dnEGFR (Dn) or 1 μg of vector (Vec). Forty-eight hours later, cells were harvested and either placed in suspension or plated on FN. The level of Erk activation was measured by immunoblotting of HA immunoprecipitates with an anti-P-Erk antibody. Total levels of Erk2 were measured by immunoblotting with an anti-Erk antibody. The level of dnEGFR expressed (Tf Dn) compared to endogenous EGFR expression (En Wt) was measured by immunoblotting cell lysates with an anti-EGFR antibody. (H) CV1 cells were infected with a virus containing an empty pLPCX vector (Vec) or a virus expressing GFP-FRNK. Erk activation in the vector- or GFP-FRNK-expressing cells was monitored in cell lysates with a phosphospecific anti-Erk antibody. Total levels of Erk in the immunoprecipitates were analyzed by immunoblotting with anti-Erk antibodies. Levels of GFP-FRNK expression were monitored by immunoblotting with an anti-FRNK antibody. Levels of endogenous FAK are also shown.