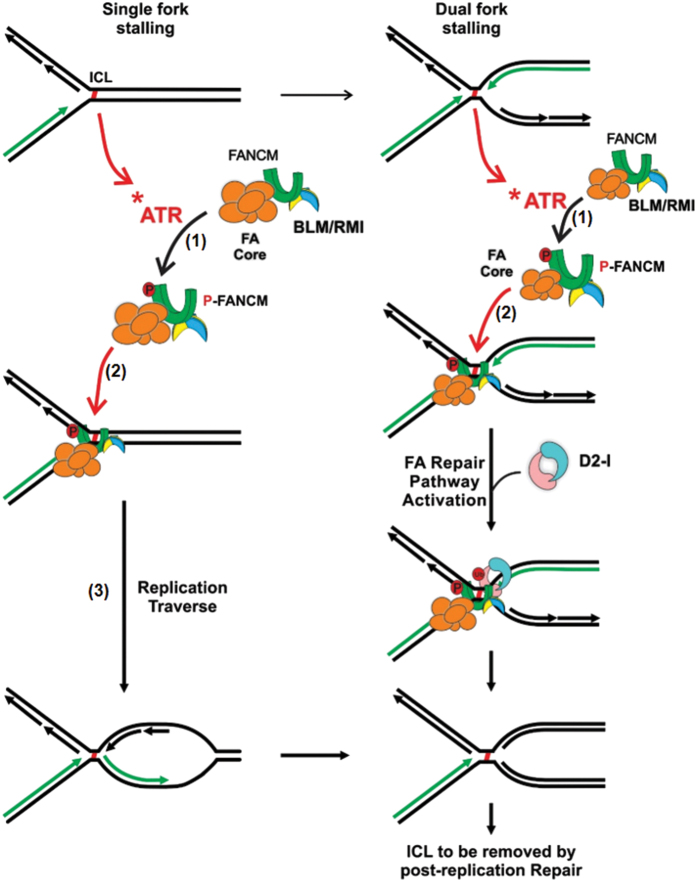
Figure 7.
A model on how BLM complex promotes FANCM recruitment to stalled forks and replication traverse of ICLs. A cartoon illustrates that the recruitment of FANCM to stalled forks is an event downstream of ATR but upstream of both FA repair pathway or the replication traverse pathway to repair or traverse ICLs. Two different scenarios when replication forks collide with ICLs (single fork-stalling and double-folk stalling) [30] are shown. The stalled single forks can continue past ICLs in the FANCM-dependent traverse pathway [30], whereas the stalled double-forks can initiate Fanconi anemia pathway to repair the ICLs [73]. BLM complex can act at multiple steps in the two pathways. It may enhance FANCM hyperphosphorylation (step 1) by direct protein–protein interactions. Alternatively, it may promote FANCM recruitment to stalled forks using its DNA binding and helicase activity (step 2). Moreover, it may stimulate the downstream traverse reaction using BLM helicase activity (step 3). FANCM is required for recruitment of FA core complex (marked by FANCA) and BLM to stalled forks [35]. Because FANCM, BLM and FA core complex form a highly stable multi-subunit complex [7, 18, 19, 23, 35], they may be co-recruited to stalled forks. Their recruitment depends on DNA translocase activity of FANCM and helicase activity of BLM, which may explain the observation that FANCM and BLM mutually depend on each other for their recruitment [35] (this study), and FA core complex depends on FANCM for its recruitment [46]. ‘D2-I’ refers to the FANCD2–FANCI complex, which is monoubiquitinated by the FA core complex in response to replication stress. The ubiquitination is marked by ‘Ub’, hyperphosphorylation is marked by ‘P’. FA, Fanconi anemia; ICL, interstrand crosslink; MMC, mitomycin C.