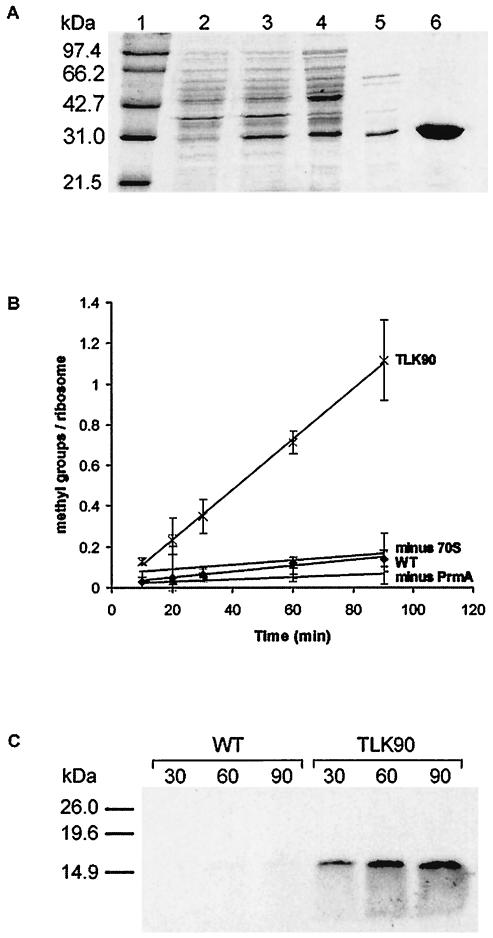
FIG. 3.
(A) Purification of cloned His6-tagged T. thermophilus L11 methyltransferase, PrmA, from E. coli BL21(DE3) carrying plasmid pET30bprmA. Lane 1, molecular size markers (sizes are indicated); lane 2, whole-cell lysate prior to induction; lane 3, whole-cell lysate after 2-h induction with IPTG; lane 4, cleared lysate after DNase treatment; lane 5, supernatant after 30-min incubation of lysate at 65°C; lane 6, purified PrmA after Ni-NTA purification, desalting, and concentration. (B). In vitro methylation of ribosomes from wild-type HB8 and the prmA null mutant, TLK90. Methylation assays were performed over a 90-min time course. ♦, ribosomes from wild-type HB8 (WT); ×, ribosomes from TLK90; +, reaction without ribosomes (minus 70S); −, ribosomes from TLK90 without the enzyme (minus PrmA). The results shown were obtained from at least three independent experiments. (C) 3H-labeled products after in vitro methylation of ribosomes from wild-type HB8 (WT) and the prmA null mutant (TLK90). Methylation assays were performed, with samples being removed after 30, 60, and 90 min and electrophoresed through a sodium dodecyl sulfate-15% polyacrylamide gel (1 pmol equivalent of ribosomes per lane). After fixing in a 10% methanol-acetic acid solution, the gels were incubated for 20 min in Enlightning rapid autoradiography enhancer (Perkin-Elmer), dried, and exposed to film for 72 h. The positions of molecular mass markers on the gel are indicated.