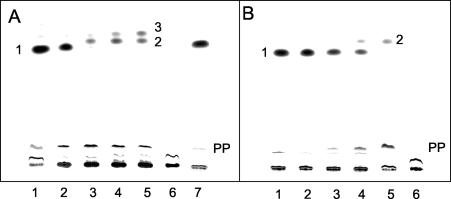
FIG. 2.
Conversion of [ring-14C]benzoyl-CoA to radioactively labeled products by cell extracts of Azoarcus sp. strain CIB or partially purified benzoyl-CoA reductase from T. aromatica. The anaerobic assay mixture contained CoA, ATP, and [ring-14C] benzoate. Benzoyl-CoA was formed enzymatically by preincubation with the partially purified benzoate-CoA ligase of T. aromatica (see Materials and Methods). Samples were taken at the indicated times and analyzed by TLC, and autoradiographs are shown. (A) Assay with cell extracts from Azoarcus sp. strain CIB. Lanes 1 to 5, samples taken after 5 s, 1 min, 3 min, 5 min, and 10 min of incubation, respectively; lane 6, the same as lane 5 but without alkaline hydrolysis; lane 7, assay without a cell extract from Azoarcus sp. strain CIB after 10 min of incubation. 1, [14C]benzoate; 2 and 3, free acids of the putative diene and monoene products; PP, free acids of more-polar products. (B) Assay with partially purified benzoyl-CoA reductase from T. aromatica. Lanes 1 to 5, samples taken after 5 s, 1 min, 3 min, 5 min, and 10 min of incubation, respectively; lane 6, the same as lane 5 but without alkaline hydrolysis. 1, [14C]benzoate; 2, free acid of the diene product; PP, free acids of more-polar products.