Fig. 1.
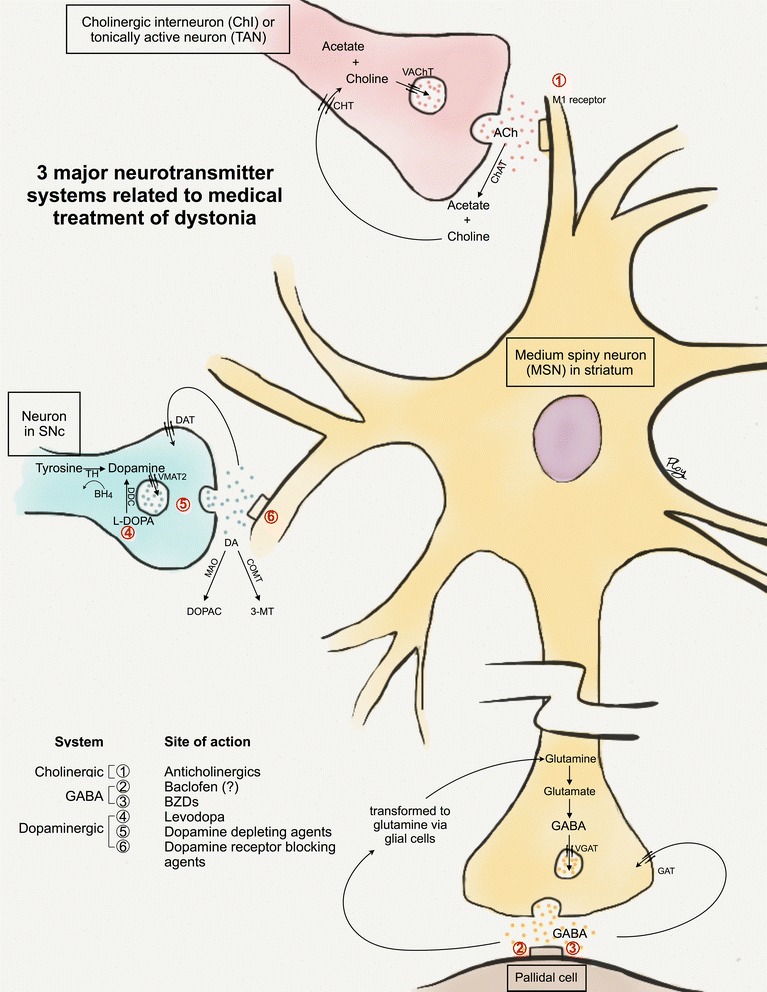
The three major neurotransmitters in dystonia. This figure illustrates the three neurotransmitters in the striatum (cholinergic [in pink], GABAergic [in yellow and brown] and dopaminergic [in blue]), their processes at synaptic levels and affected targets. Of note, other neurotransmitters such as cannabinoids and serotonin may also play a role in dystonia but are not shown here. 1) Cholinergic system. Giant asypiny or cholinergic interneurons (ChIs; in pink), also referred to as tonically active neurons (TANs), are a main cholinergic input to medium spiny neurons (MSNs; in yellow) in the striatum. At the synaptic level, ACh is synthesized in presynaptic terminals by acetylation of choline, catalyzed by the enzyme choline acetyltransferase (ChAT). ACh is then transported into vesicles by the vesicular ACh transporter (VAChT). After ACh is released at synaptic clefts, it binds to muscarinic (M1-4 subtypes) and/or nicotinic receptors in order to have further action downstream. The remaining ACh at the synaptic cleft is subsequently metabolized by acetylcholinesterase (AChE) into acetate and choline. The latter is taken up into the presynaptic terminal by the choline transporter (CHT). 2) GABAergic system. GABA is present widely in neurons subserving basal ganglia circuitry including the MSNs, and both internal and external segments of the globus pallidus. In this figure, only the synapse between the MSN and the pallidal cell (in brown) is demonstrated. At the synaptic level, GABA is synthesized from glutamate in presynaptic terminals. It is then packed into vesicles via the vesicular GABA transporter (VGAT) before being released into synaptic clefts. GABA subsequently binds to postsynaptic receptors. The remaining GABA at the synaptic clefts is transported back to presynaptic terminals by two methods: 1) direct reuptake by GABA transporters (GAT) at presynaptic terminals 2) indirect transport via adjacent glial cells requiring transformation to glutamine prior to returning to presynaptic terminals. 3) Dopaminergic system. The MSNs also receive dopaminergic input from neurons in the substantia nigra pars compacta (SNc) via the nigrostriatal pathway (in blue). At the synaptic level, dopamine is synthesized in presynaptic terminals from tyrosine by the enzyme tyrosine hydroxylase (TH) requiring tetrahydrobiopterin (BH4) as a cofactor. Dopamine (DA) and other monoamines are packaged into vesicles in presynaptic terminals by the enzyme vesicular monoamine transporter 2 (VMAT2). The monoamines are then released to synaptic clefts and bind to postsynaptic receptors including dopamine receptors (D1-5). Dopamine at synaptic clefts is degraded by the enzymes monoamine oxidase (MAO) and cathechol-O-methyl transferase (COMT) into 3,4-dihydroxyphenylacetic acid (DOPAC) and 3-methoxytyramine (3-MT) respectively. The remaining dopamine is subsequently transported back to presynaptic terminals by the dopamine transporters (DAT). The prototypic medications affecting each neurotransmitter systems and their sites of action are listed at the left lower corner. Anticholinergics act postsynaptically as muscarinic receptor antagonists, particularly at M1 receptors. Baclofen is a GABAB receptor agonist. In the spinal cord, it acts at both presynaptic (excitatory glutamatergic neurons) and postsynaptic (of inhibitory interneurons) terminals. However, its sites of action in the basal ganglia (presynaptic vs. postsynaptic or both) remain unclear (shown as “?”). Benzodiazepines (BZDs) bind to GABAA receptors, leading to increased frequency of chloride channel opening and thereby inhibitory signals. Levodopa (L-DOPA) is converted to dopamine in presynaptic terminals by the enzyme DOPA decarboxylase (DDC). Dopamine depleting agents such as tetrabenazine (TBZ) acts at presynaptic terminals by inhibiting the VMAT2 enzyme which then impairs dopamine transport into vesicles. Dopamine receptor blocking agents (DRBAs), in contrast, acts postsynaptically by blocking dopamine receptors
