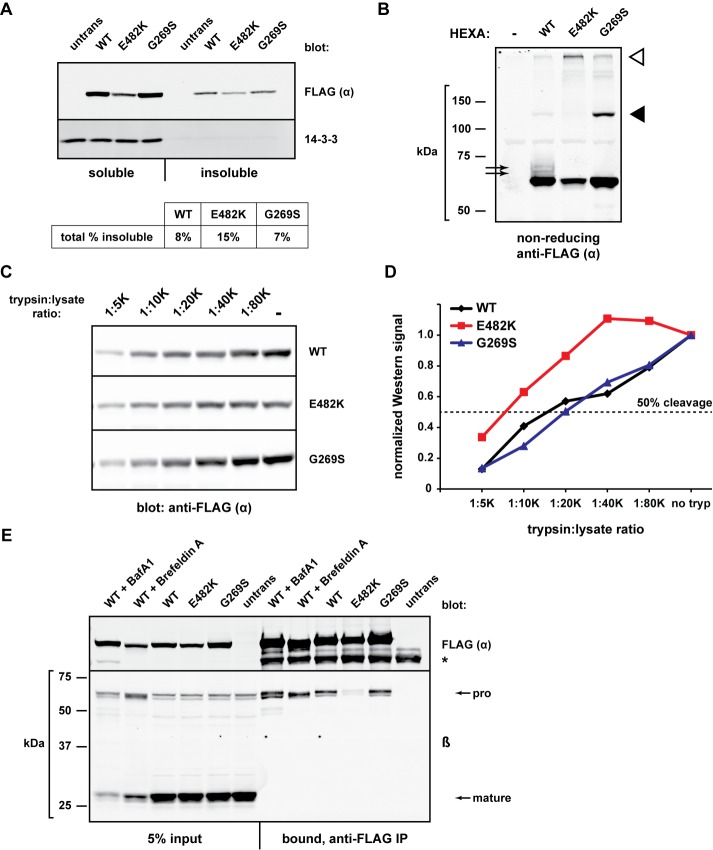
FIGURE 3:
Conformational and biochemical differences among α mutants. (A) The indicated FLAG-HEXA constructs were expressed in HEK293T cells for 20 h and harvested in lysis buffer containing 1% NP-40. Insoluble material was resuspended in 3% SDS, heated, and sonicated. Samples of the NP-40–soluble (5% of total) and NP-40–insoluble (10% of total) were subjected to gel electrophoresis and Western blotting with anti-FLAG. Band quantifications were used to determine the overall fraction of insoluble material of each α protein. (B) FLAG-HEXA constructs were transiently expressed for 24 h, and cell lysates were prepared in nonreducing Laemmli sample buffer before SDS–PAGE and immunoblot analysis. Black arrows, slower migrating species only present upon WT α expression; black arrowhead, G269S-specific band migrating at a size corresponding to a dimer; white arrowhead, species resistant to gel migration. (C) HEK293T cells expressing the indicated FLAG-HEXA constructs were harvested and lysed without protease inhibitors. Equal amounts of total protein were incubated with decreasing concentrations of trypsin (1:20 K ratio indicates 1 μg trypsin per 20,000 μg whole-cell lysate) for 1 h at 37°C. Reactions were quenched with SDS sample buffer before SDS–PAGE and Western blotting. (D) Quantification of band intensity from C, normalized to the “no trypsin” sample of each set. Fifty percent of starting material is indicated by dashed line. (E) HEK293T cells were transiently transfected with FLAG-HEXA constructs and were left untreated or treated with brefeldin A (0.5 μg/ml) or bafilomycin A1 (50 nM) for 18.5 h. Cell lysates were then subjected to IP with anti-FLAG agarose to determine the co-IP of endogenous β with FLAG-α for each mutant/condition. *, heavy chain of the anti-FLAG antibody used for IP.