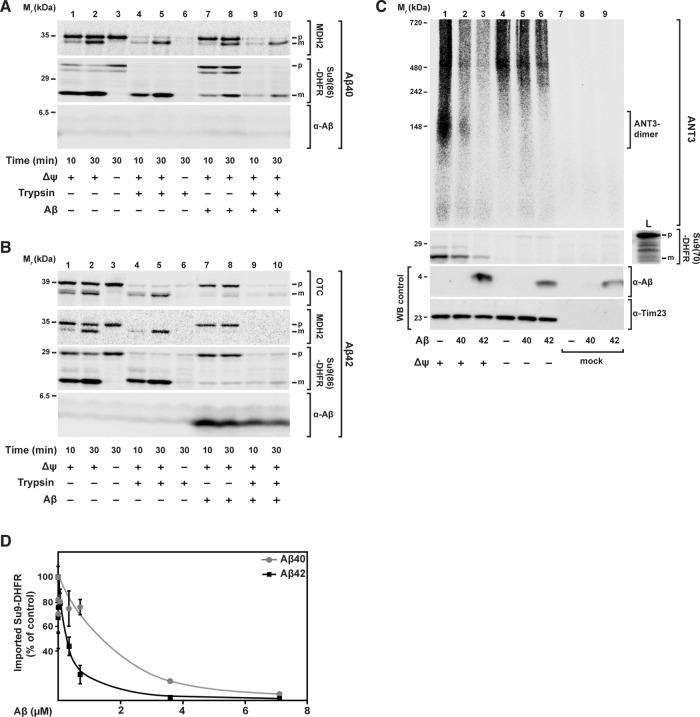
FIGURE 1:
Effect of Aβ peptides on mitochondrial import of nuclear-encoded precursor proteins. 35S‑labeled radioactive precursor proteins were incubated with energized and isolated mitochondria from HeLa cell cultures in the presence of same amounts (3.5 μM) of Aβ40 and Aβ42 peptides. (A, B) Import of the precursor proteins mitochondrial MDH2, the artificial reporter construct Su9(86)-DHFR, and OTC for the indicated incubation times. After the import reaction, half of the samples (lanes 4–6, 9, and 10) were treated with trypsin (100 μg/ml) to remove nonimported preproteins. Imported proteins were analyzed by tricine-SDS–PAGE, followed by Western blot, digital autoradiography, and immunodecoration against Aβ peptides. (C) Import of ANT3 in comparison with Su9(70)-DHFR. After import, all samples were treated with PK (50 μg/ml) and analyzed by BN- (ANT3) or SDS–PAGE (Su9(70)-DHFR), Western blot, and digital autoradiography. As control, immunodecoration against Tim23 was carried out. (D) Quantification of import-inhibitory effect of Aβ peptides. Import experiments with the precursor protein [35S]Su9(86)DHFR and different amounts of Aβ peptides (0.007–7.0 μM) were performed as described. The signals of processed and protease-resistant preprotein bands (m form) were quantified using ImageJ. The amount of imported protein in the absence of Aβ peptide was set to 100%. Mean values and SD were determined for three independent experiments. L, loading control; m, mature processed form; p, precursor protein; WB, Western blot.