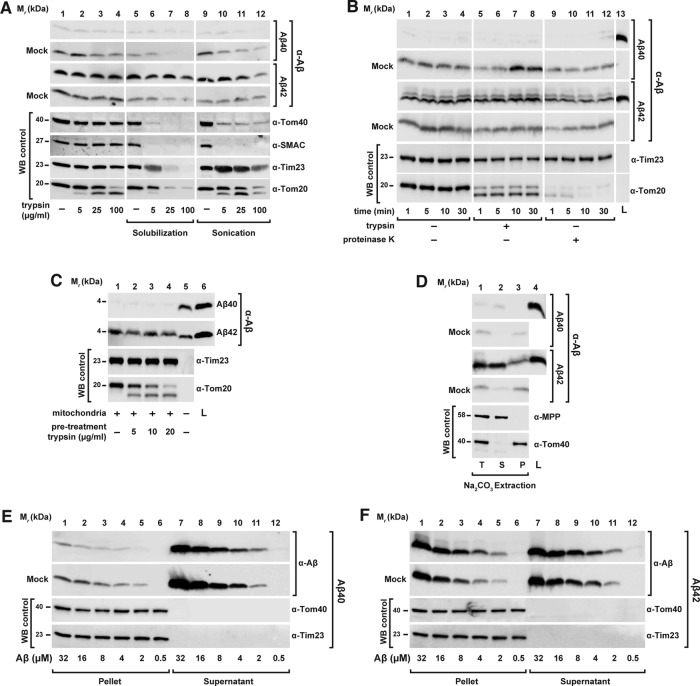
FIGURE 5:
Membrane interaction behavior of Aβ peptides. (A) Absent protease protection by mitochondria. Aβ peptides (3.5 μM) were incubated with or without (Mock) intact and energized mitochondria, followed by digestion with increasing amounts of trypsin (lanes 1–4). As controls, mitochondria were lysed by solubilization with 0.5% Triton X-100 (lanes 5–8) or by sonication (lanes 9–12) before addition of the protease. All samples underwent TCA precipitation. (B) Intrinsic protease resistance. Aβ peptides (3.5 μM) were incubated with or without (Mock) intact and energized mitochondria, followed by digestion with 100 μg/ml trypsin (lanes 5–8) and 100 μg/ml PK for different times as indicated. (C) Dependence of the interaction between Aβ peptides and isolated mitochondria on peripheral OMM receptors. Isolated mitochondria were pretreated with the indicated trypsin concentrations to digest exposed OMM proteins. After trypsin inactivation, isolated mitochondria were reisolated and incubated in an energized buffer with Aβ peptides (3.5 μM). (D) Alkaline extraction of Aβ peptides from mitochondria and mock samples. Aβ peptides (3.5 μM) were incubated in the presence or absence (Mock) of isolated and energized mitochondria. After reisolation, mitochondria and mock samples were subjected to alkaline extraction as described in Material and Methods. (E, F) Titration of Aβ peptide binding to mitochondria. Increasing concentrations of Aβ40 (E) and Aβ42 (F) peptides were incubated for 30 min in the presence or absence (Mock) of energized mitochondria and separated in insoluble (Pellet) and soluble (Supernatant) fractions. All samples were analyzed by tricine-SDS–PAGE and Western blot. As control, immunodecoration against the endogenous mitochondrial proteins SMAC (intermembrane space), MPP (matrix) and Tom40 (OMM) was carried out. L, loading control; P, pellet; S, supernatant; T, total; WB, Western blot.