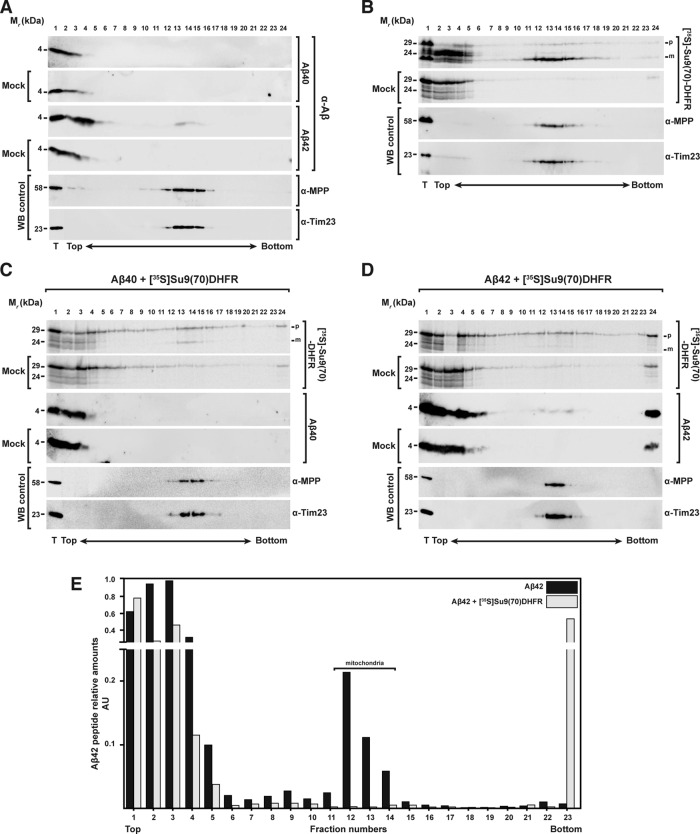
FIGURE 6:
Analysis of the interaction between Aβ peptides and mitochondrial precursor proteins with mitochondria through density gradient centrifugation. (A) Sucrose gradient centrifugation of 3.5 μM Aβ40 (top) and Aβ42 (bottom) incubated with and without (Mock) isolated and energized mitochondria. (B) As control, a sucrose gradient of precursor protein [35S]Su9(70)DHFR incubated with or without (Mock) isolated and energized mitochondria in the absence of Aβ peptides was performed. (C, D) Sucrose gradients with or without (Mock) mitochondria incubated with precursor protein [35S]Su9(70)DHFR in the presence of Aβ40 (C) or Aβ42 (D). Density gradient fractionations were performed as reported in Materials and Methods. Samples were analyzed by tricine-SDS–PAGE and Western blot. As control, immunodecorations against MPP and Tim23 were used. (E) Quantification of the Aβ42 band intensities incubated with mitochondria in the absence (A) or presence (D) of precursor protein [35S]Su9(70)DHFR. Each value is the ratio between the intensity of the Aβ42 band in each fraction and the total sample (T). m, mature form of the preprotein; p, precursor form; WB, Western blot.