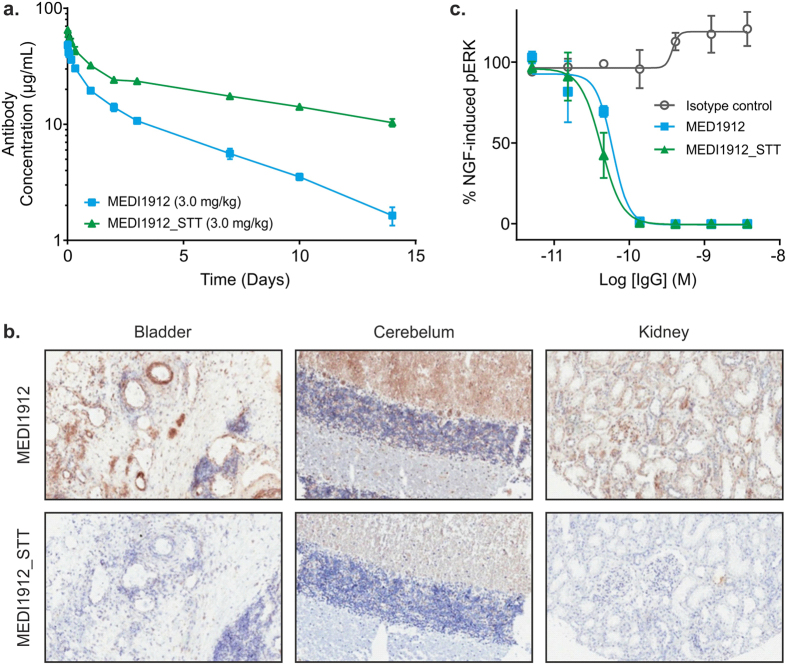
Figure 5. Effect of STT mutations on MEDI1912 pharmacokinetics, tissue specificity and functional potency.
(a) MEDI1912 and MEDI1912_STT PK profiles following intravenous administration of a 3 mg/kg dose to rats (n = 3). (b) Human bladder, cerebellum and kidney tissue immunohistochemically stained with MEDI1912 and MEDI1912_STT at 0.18 μg/mL. Significant staining was demonstrated by MEDI1912, showing strong staining in connective tissue, smooth muscle (around blood vessels) and other areas that is consistent with non-specific staining. MEDI1912_STT showed no evidence of staining in any tissues evaluated. (c) MEDI1912_STT retains functional potency in vitro compared with MEDI1912 in a phospho-ERK activation assay. Data points represent the mean ± SD.