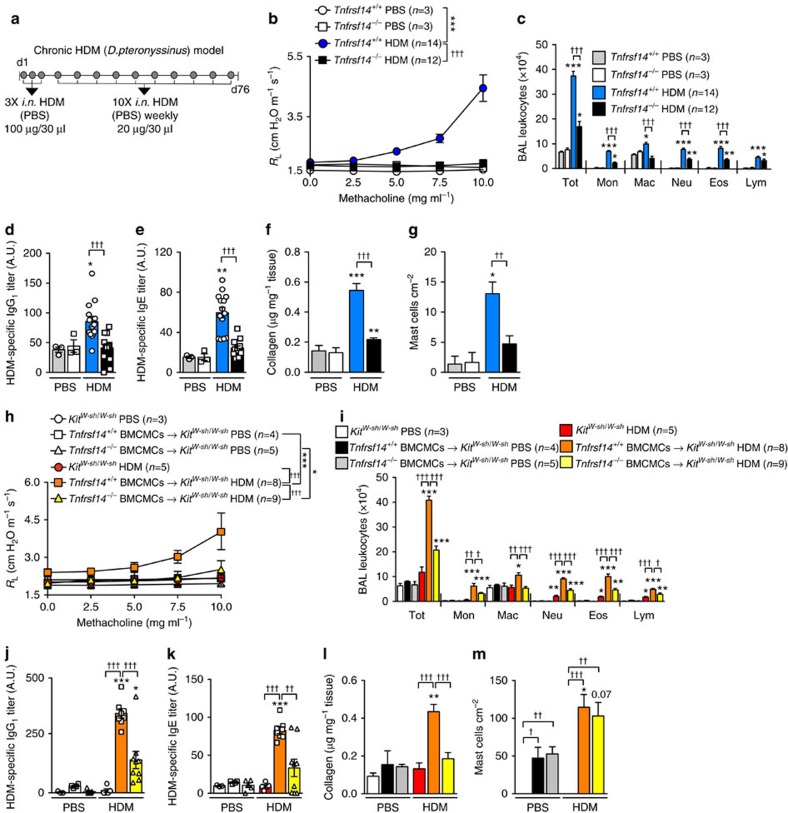
Figure 8. MC TNFRSF14 contributes to HDM-induced asthma pathology.
(a) Protocol for inducing HDM (D. pteronyssinus) sensitization and HDM-induced airway pathology. Tnfrsf14−/− and littermate control Tnfrsf14+/+ mice were sensitized with three intranasal (i.n.) administrations of HDM followed by 10 weekly i.n. challenges with HDM; control mice were mock-sensitized with PBS, followed by 10 weekly intranasal (i.n.) challenges with PBS. (b) Changes in RL induced by aerosolized methacholine, (c) numbers of leukocytes in BAL fluid, (d,e), titers of plasma HDM-specific IgG1 (d) and IgE (e), (f) lung collagen and (g) numbers of lung MCs, 24 h after the tenth HDM or PBS challenge in Tnfrsf14−/− and Tnfrsf14+/+ mice. (h–m) Changes in RL (h) induced by aerosolized methacholine, (i) numbers of leukocytes in BAL fluid, (j,k), titers of plasma HDM-specific IgG1 (j) and IgE (k), (l) lung collagen and (m) numbers of lung MCs, 24 h after the tenth HDM (or PBS) challenge in MC-deficient KitW-sh/W-sh mice and in KitW-sh/W-sh mice engrafted with Tnfrsf14+/+ or Tnfrsf14−/− BMCMCs. Three-to-fourteen female mice per group were used as indicated. Results are pooled from at least two independent experiments, each of which gave similar results. The data in b–m (shown as mean+or±s.e.m.) were assessed for statistical significance using a two-tailed Student's t-test or a two-way analysis of variance (ANOVA) test. Asterisks indicate statistical significance of differences between PBS-treated and corresponding Ag-treated groups; daggers indicate statistical significance between indicated groups. * or †P<0.05; ** or ††P<0.01; *** or †††P<0.001.