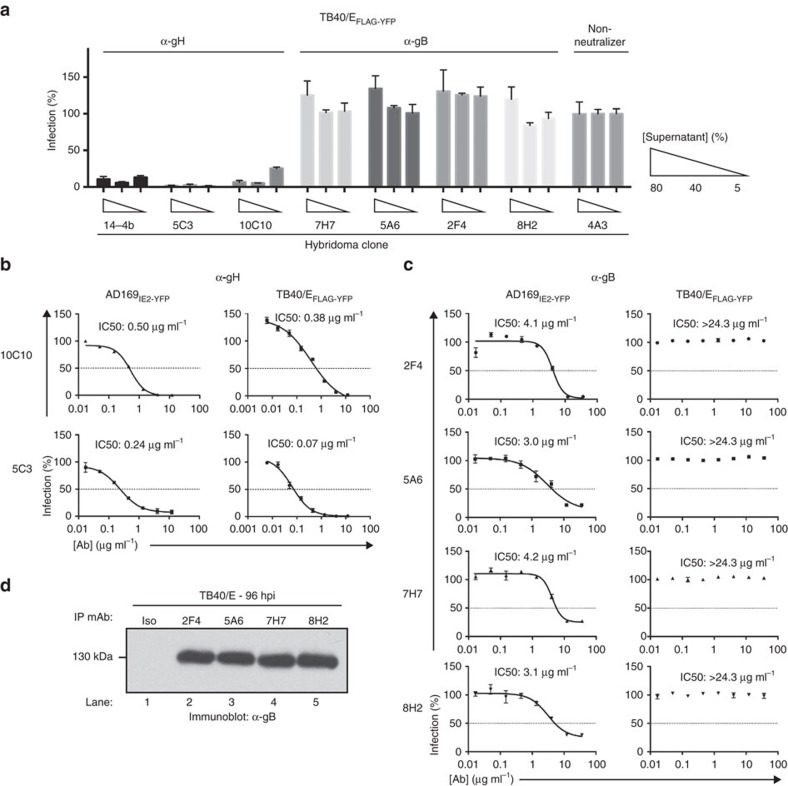
Figure 3. Examination of the neutralizing capacity of anti-CMV mAbs.
(a) Hybridoma supernatant from the 6 CMV-neutralizing mAbs was tested at 3 concentrations (5, 40 and 80%) for their ability to inhibit TB40/EFLAG-YFP infection of MRC5 cells. Supernatant from the neutralizing anti-gH mAb 14-4b and supernatant from the non-neutralizing hybridoma clone 4A3 were utilized as controls. Experiments were performed in technical triplicates and s.d. is depicted. (b) Anti-gH mAbs 10C10 and 5C3 were pre-incubated with AD169IE2-YFP (left panel) and TB40/EFLAG-YFP (right panel) at 8 concentrations (0.01–12 μg ml−1) and infection levels of MRC5 cells was subsequently measured. (c) Anti-gB mAbs 2F4, 5A6, 7H7 and 8H2 were pre-incubated with AD169IE2-YFP (left panel) and TB40/EFLAG-YFP (right panel) at 8 concentrations (0.01–12 μg ml−1) and infection levels of MRC5 cells was subsequently measured. Non-linear regression analysis was performed and the half maximal inhibitory concentration (IC50) was calculated for all antibodies. (d) MRC5 cells infected with TB40/E were harvested at 96 hpi and total cell lysates were exposed to the anti-gB antibodies. Recovered immune complexes were resolved by SDS–PAGE and exposed to anti-gB immunoblot (lanes 1–5). Relative molecular mass markers are indicated. Experiments for (a–c) were performed in technical triplicate and s.d. is depicted. Respective virus neutralization experiments were replicated four times each.