Abstract
To investigate genetic mechanisms of high altitude adaptations of native mammals on the Tibetan Plateau, we compared mitochondrial sequences of the endangered Pantholops hodgsonii with its lowland distant relatives Ovis aries and Capra hircus, as well as other mammals. The complete mitochondrial genome of P. hodgsonii (16,498 bp) revealed a similar gene order as of other mammals. Because of tandem duplications, the control region of P. hodgsonii mitochondrial genome is shorter than those of O. aries and C. hircus, but longer than those of Bos species. Phylogenetic analysis based on alignments of the entire cytochrome b genes suggested that P. hodgsonii is more closely related to O. aries and C. hircus, rather than to species of the Antilopinae subfamily. The estimated divergence time between P. hodgsonii and O. aries is about 2.25 million years ago. Further analysis on natural selection indicated that the COXI (cytochrome c oxidase subunit I) gene was under positive selection in P. hodgsonii and Bos grunniens. Considering the same climates and environments shared by these two mammalian species, we proposed that the mitochondrial COXI gene is probably relevant for these native mammals to adapt the high altitude environment unique to the Tibetan Plateau.
Key words: tibetan antelope, mitochondrial genome, adaptation, COXI
Introduction
As the most prominent terrestrial highland on the earth, the Tibetan Plateau enacts great effects on global climate and biosphere. Its fauna and flora prospering on the plateau are constantly challenged by the harsh environment of hypoxia, low temperature, high solar radiation, and lack of biological production. Native animals of the Tibetan Plateau, surviving over thousands of years on the highland, have developed various physiological, behavioral, and morphological strategies to cope with these environmental challenges; some of the changes are certainly attributable to phenotypic plasticity and others are genetic. The genomic mean for discovering inheritable changes in a species is to sequence its genomes, including both nuclear and organellar genomes.
Pantholops hodgsonii (Tibetan antelope or chiru) is an excellent representative of the native mammalian species, which has perfectly adapted to the Tibetan Plateau. Living at elevations from 3,700 to 5,500 meters (1), the antelope can run for hours at a spectacular speed. Such high speed and stamina indicate that it is very effective in utilizing limited oxygen supply and other energy resources on the plateau. Therefore, we chose P. hodgsonii as an initial subject to look for any adaptive genetic changes, at both DNA and protein levels, and molecular mechanisms for high altitude adaptations of native mammals that thrive on the Tibetan Plateau.
During the past few decades, mitochondrial DNA (mtDNA) has been widely used in studies of evolutionary biology and population genetics. As the locality of energy metabolism and hosting unique genetic material, mitochondrion and its genome are particularly interesting for high altitude biology. Mammalian mitochondrial genome is a circular, double-stranded molecule with a length of about 16 Kb. In general, it contains 13 protein-coding genes, 2 rRNA genes, 22 tRNA genes, and a non-coding control region 2., 3., 4.. The thirteen proteins encoded by the genome are all related to oxide metabolism.
In the current study, we report a complete mitochondrial genome sequence from a single P. hodgsonii individual and results from comparative analysis in search of genetic outcomes in living under high altitude environments.
Results
General features of the P. hodgsonii mitochondrial genome
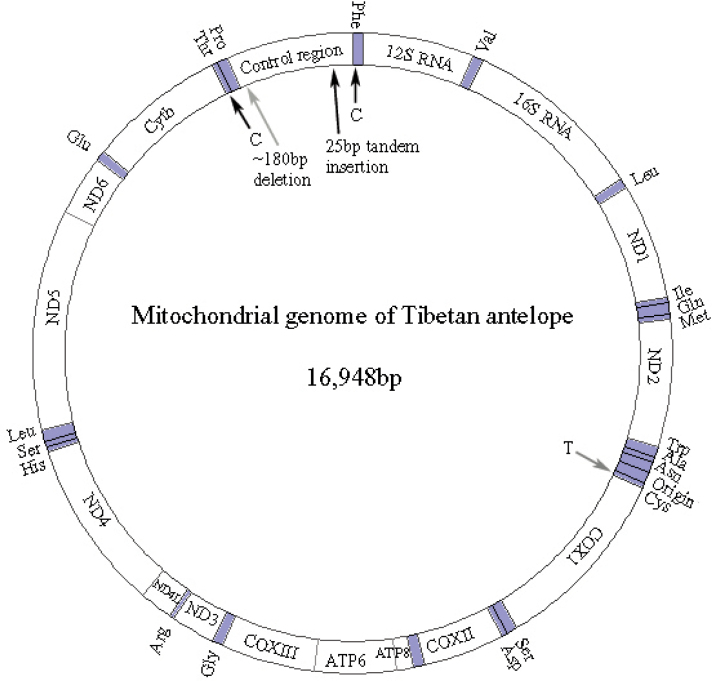
The P. hodgsonii mitochondrial genome is 16,498 bp long, shorter than those of Ovis aries (NC_001941) and Capra hircus (NC_005044), which are 16,616 bp and 16,640 bp in length, respectively, yet longer than that of Bos taurus (NC_001567), Bos indicus (NC_005971), and Bubalus bubalis (NC_006295), which are 16,338 bp, 16,339 bp, and 16,359 bp in length, respectively. The size differences are resulted from different lengths of the control region among these species. Nucleotide composition analysis revealed that the P. hodgsonii mitochondrial genome is biased towards AT (A 33.59%, T 26.87%, G 13.11%, and C 26.41%); such an AT content is lower than those of O. aries and C. hircus. The P. hodgsonii mitochondrial genome encodes 13 proteins, 2 rRNAs, and 22 tRNAs (Figure 1). Eight tRNA genes and one protein gene are located on the light strand (Table 1). And the number of polymorphic sites at protein coding genes and RNA genes in the mitochondrial genomes of P. hodgsonii, C. hircus and O. aries is shown in Table 2.
Fig. 1.
Annotation of the Pantholops hodgsonii mitochondrial genome. The original figure was from Bae et al. (30). ND1 to ND6 refer to NADH subunits; COXI, COXII, COXIII refer to cytochrome c oxidase subunits; ATP6 and ATP8 refer to ATPase6 and ATPase8; the tRNA genes are denoted by shade and are noted accordingly; Origin refers to Origin of L-strand replication. The ATPase8 and ATPase6 genes are overlapped for 40 bp. Some bases between genes are too few to be denoted in the figure.
Table 1.
Components of P. hodgsonii Mitochondrial Genome
| Gene | Direction | Nucleotide number | Start codon | Stop codon |
|---|---|---|---|---|
| tRNA Phe | F | 1–69 | ||
| 12S rRNA | F | 70–1,026 | ||
| tRNA Val | F | 1,027–1,093 | ||
| 16S rRNA | F | 1,094–2,659 | ||
| tRNA Leu | F | 2,663–2,737 | ||
| NADH1 | F | 2,740–3,694 | ATG | TAa |
| tRNA Ile | F | 3,696–3,764 | ||
| tRNA Gln | R | 3,762–3,833 | ||
| tRNA Met | F | 3,836–3,904 | ||
| NADH2 | F | 3,905–4,946 | ATA | Taa |
| tRNA Trp | F | 4,947–5,013 | ||
| tRNA Ala | R | 5,015–5,083 | ||
| tRNA Asn | R | 5,085–5,157 | ||
| Origin of L-strand replication | R | 5,158–5,189 | ||
| tRNA Cys | R | 5,190–5,256 | ||
| tRNA Tyr | R | 5,257–5,324 | ||
| COXI | F | 5,326–6,870 | ATG | TAA |
| tRNA Ser | R | 6,868–6,936 | ||
| tRNA Asp | F | 6,944–7,011 | ||
| COXII | F | 7,013–7,696 | ATG | TAA |
| tRNA Lys | F | 7,700–7,767 | ||
| ATPase8 | F | 7,769–7,969 | ATG | TAA |
| ATPase6 | F | 7,930–8,610 | ATG | Taa |
| COXIII | F | 8,610–9,393 | ATG | Taa |
| tRNA Gly | F | 9,394–9,462 | ||
| NADH3 | F | 9,463–9,809 | ATA | TAa |
| tRNA Arg | F | 9,810–9,878 | ||
| NADH4L | F | 9,879–10,175 | ATG | TAA |
| NADH4 | F | 10,169–11,546 | ATG | Taa |
| tRNA His | F | 11,547–11,616 | ||
| tRNA Ser | F | 11,617–11,676 | ||
| tRNA Leu | F | 11,678–11,747 | ||
| NADH5 | F | 11,748–13,568 | ATA | TAA |
| NADH6 | R | 13,555–14,081 | ATG | TAA |
| tRNA Glu | R | 14,080–14,148 | ||
| Cytochrome b | F | 14,153–15,292 | ATG | AGA |
| tRNA Thr | F | 15,296–15,366 | ||
| tRNA Pro | R | 15,366–15,431 | ||
| Control region | 15,432–16,498 |
Table 2.
Number of Polymorphic Sites at Protein Coding Genes and RNA Genes
| Location | Gene | Number of mutations |
|||
|---|---|---|---|---|---|
| All | P. hodgsonii | C. hircus | O. aries | ||
| 1 | tRNA-Phe | 9 | 4 | 2 | 1 |
| 70 | 12s rRNA | 101 | 39 | 29 | 31 |
| 1,027 | tRNA-Val | 10 | 5 | 3 | 2 |
| 1,094 | 16s rRNA | 160 | 53 | 56 | 45 |
| 2,663 | tRNA-Leu | 4 | 4 | ||
| 2,740 | NU1M | 149 | 53 | 48 | 42 |
| 3,762 | tRNA-Gln (L) | 1 | 1 | ||
| 3,836 | tRNA-Met | 1 | 1 | ||
| 3,905 | NU2M | 168 | 59 | 63 | 43 |
| 4,947 | tRNA-Trp | 3 | 3 | ||
| 5,015 | tRNA-Ala (L) | 4 | 1 | 1 | 2 |
| 5,085 | tRNA-Asn (L) | 4 | 2 | 2 | |
| 5,190 | tRNA-Cys (L) | 1 | 1 | ||
| 5,257 | tRNA-Tyr (L) | 4 | 2 | 1 | 1 |
| 5,326 | COXI | 241 | 100 | 65 | 71 |
| 6,868 | tRNA-Ser (L) | 4 | 1 | 3 | |
| 6,944 | tRNA-Asp | 3 | 1 | 1 | 1 |
| 7,013 | COXII | 106 | 35 | 36 | 32 |
| 7,700 | tRNA-Lys | 12 | 4 | 3 | 4 |
| 7,769 | ATP8 | 39 | 13 | 12 | 11 |
| 7,930 | ATP6 | 131 | 44 | 45 | 39 |
| 8,610 | COXIII | 148 | 59 | 56 | 30 |
| 9,394 | tRNA-Gly | 4 | 1 | 2 | |
| 9,463 | NU3M | 57 | 25 | 13 | 17 |
| 9,810 | tRNA-Arg | 5 | 1 | 3 | 1 |
| 9,879 | NU4L | 45 | 10 | 10 | 23 |
| 10,169 | NU4M | 273 | 101 | 74 | 90 |
| 11,547 | tRNA-His | 8 | 3 | 4 | |
| 11,617 | tRNA-Ser | 10 | 5 | 2 | 3 |
| 11,678 | tRNA-Leu | 2 | 2 | ||
| 11,748 | NU5M | 365 | 126 | 110 | 121 |
| 13,555 | NU6M (L) | 69 | 19 | 25 | 24 |
| 14,080 | tRNA-Glu (L) | 6 | 3 | 2 | 1 |
| 14,153 | CYB | 185 | 60 | 62 | 57 |
| 15,296 | tRNA-Thr | 11 | 6 | 4 | 1 |
| 15,366 | tRNA-Pro (L) | 5 | 4 | 1 | |
Number of polymorphic sites at protein coding genes and RNA genes in the mitochondrial genomes of P. hodgsonii, C. hircus and O. aries. The column “All” refers to the number of sites at which all three species are different; the column “P. hodgsonii” refers to number of the sites at which P. hodgsonii is different from the other two species.
Protein coding genes
There are 13 protein-coding genes in the P. hodgsonii mitochondrial genome. Among these genes, eight use ATG as start codon and three (NADH2, NADH3, NADH5) use ATA as start codon. Some of these 13 protein genes are terminated with incomplete stop codons: NADH1, NADH3, and ATP6 are terminated with TA; COXIII (cytochrome c oxidase subunit III), NADH2, and NADH4 are terminated with T; the rest are terminated with TAA and AGA (Table 1). Presumably, these incomplete stop codons are accommodated post-transcriptionally in the mRNA maturation process, i.e. polyadenylation (5).
We compared protein sequences between P. hodgsonii and other mammalian species. The P. hodgsonii CYTB, ND6, ND4L, and COXII bare higher homology to those of C. hircus than O. aries and other species; Its COXI, COXIII, NU1M, NU2M, NU3M, NU4M, NU5M, and NU6M are most similar to those of O. aries than C. hircus and other species. Interestingly, ATP8 of P. hodgsonii is 93.93% identical to that of Bos grunniens whereas it shares much identity with those of other species, including those of O. aries (89.39%) and C. hircus (83.33%). The nucleotide similarity of these P. hodgsonii proteins is in general higher when compared to O. aries and C. hircus than to other species. AT contents of the P. hodgsonii protein coding genes are higher than those of human and lower than those of mouse and rat (Data not shown).
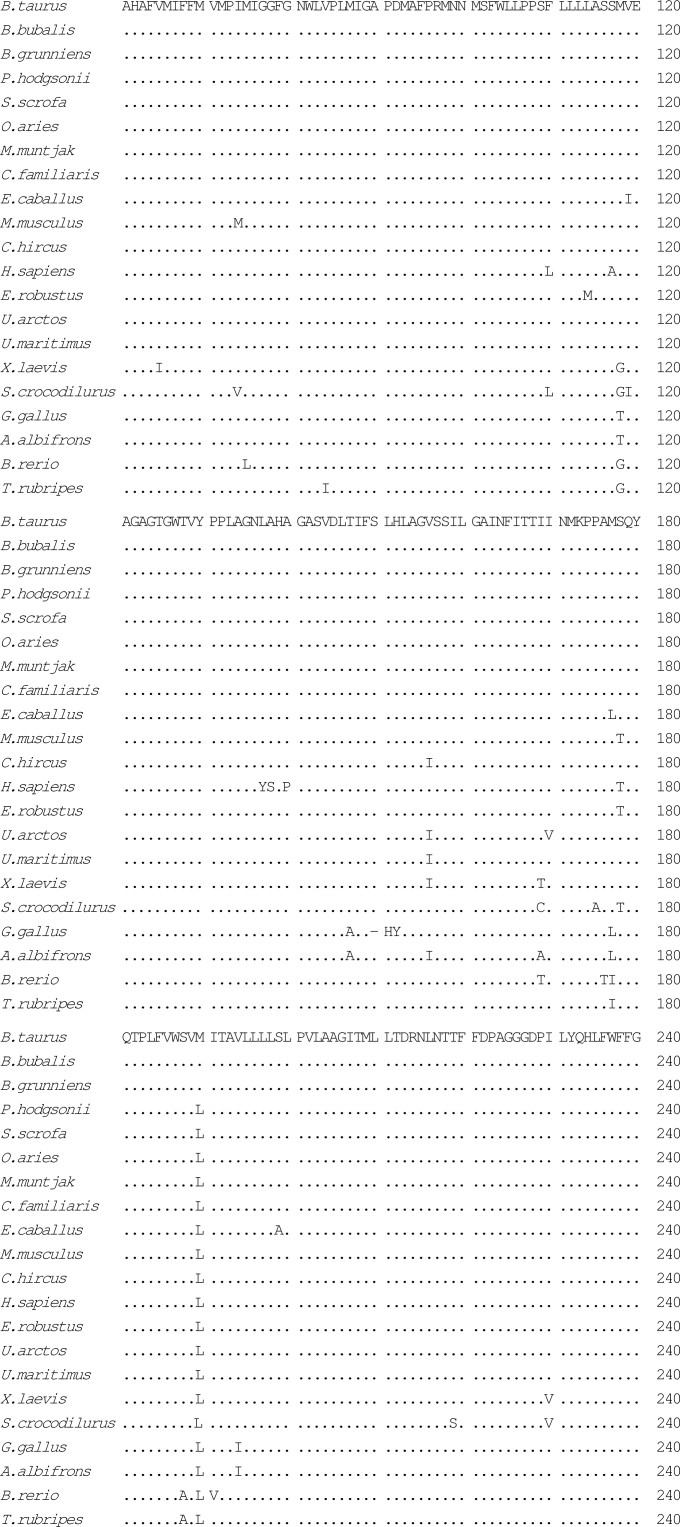
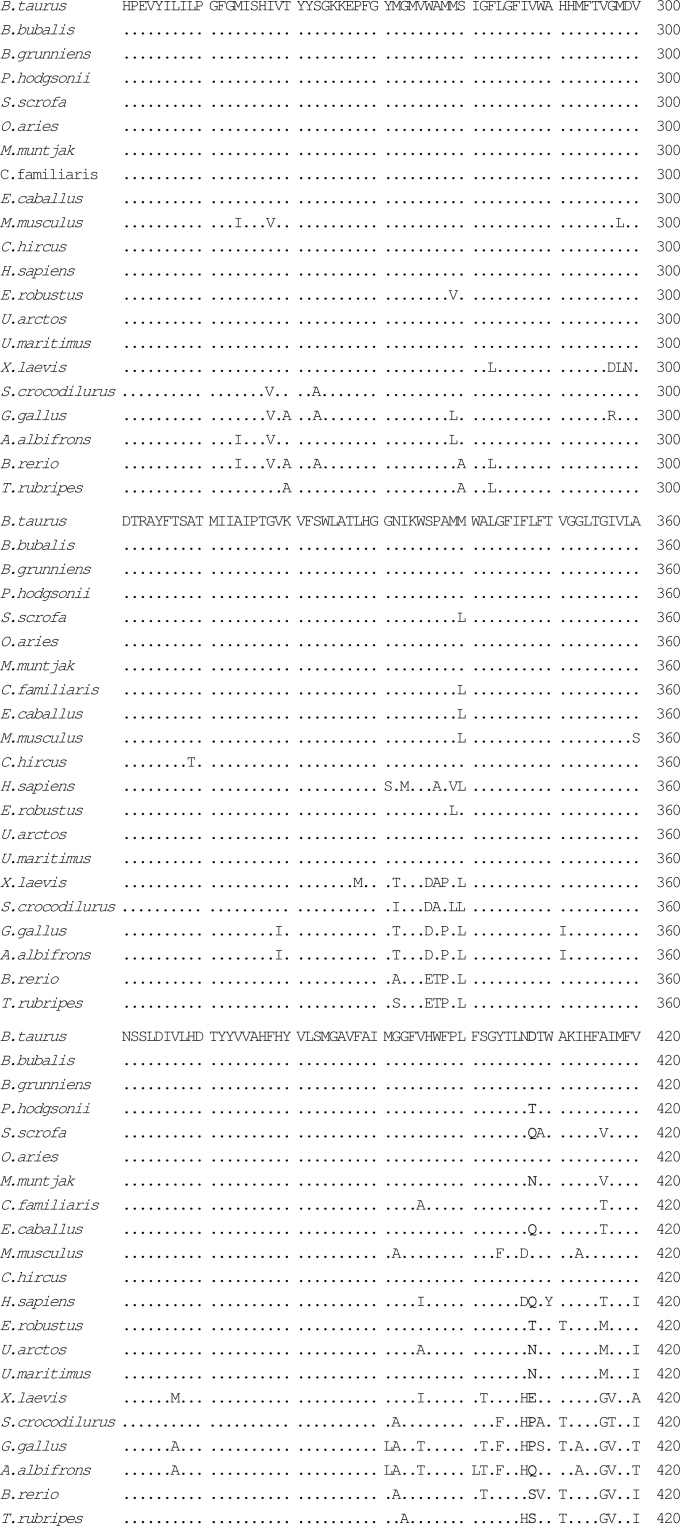
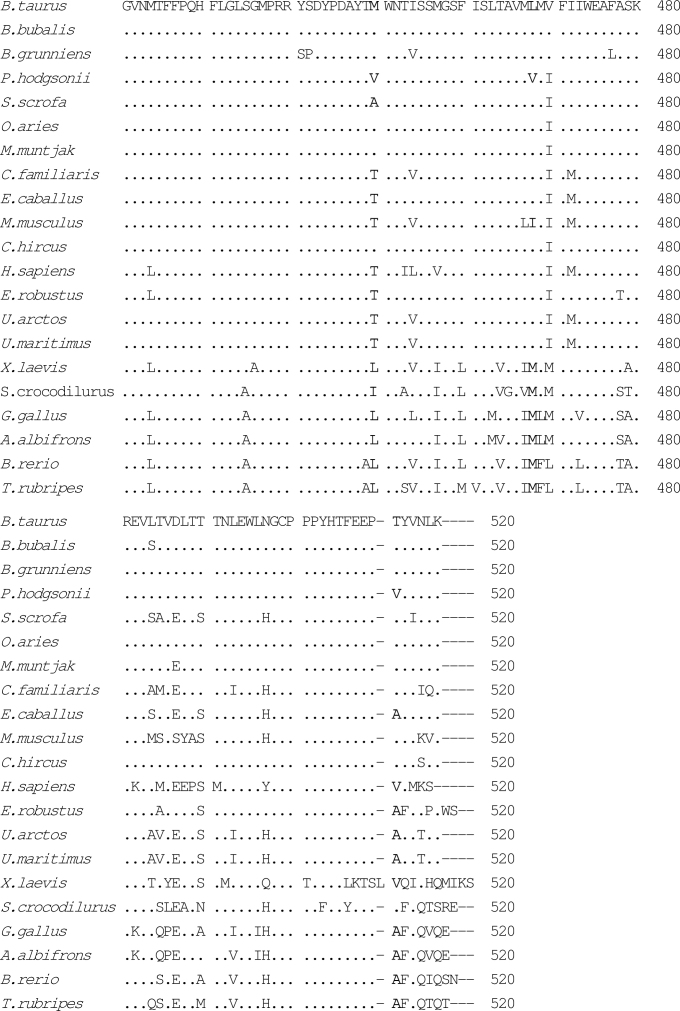
We analyzed mutations of four protein coding genes in details, including CYTB, COXI, COXII, and COXIII, among O. aries, C. hircus, B. grunniens, P. hodgsonii, and a few other species of the Bovidae family, attempting to predict possible functional implications for mutations discovered from comparative analyses. In the case of COXI, there are five mutations affecting amino acids in P. hodgsonii, four in B. grunniens, three in C. hircus, and none in O. aries, as compared with B. taurus (Table 3). The amino acid changes in P. hodgsonii and B. grunniens occur mostly at sites between 400 and 500 (Table 3, Figure 2), whereas in C. hircus the mutations were found within 150 to 512. The region from 400 to 500 constitutes the transmembrane components X, XI, and XII.
Table 3.
Amino Acid Mutations of the COXI Gene
| Species | Mutation | E/B | Neighbor rigidity | Rigidity | Volume change | Charge change | Polarity change |
|---|---|---|---|---|---|---|---|
| C. hircus | V155I | 64 | −0.2351483 | −0.34353 | 26.7 | 0 | 0 |
| A308T | 5 | −0.2854679 | −0.4675 | 27.5 | 0 | 0 | |
| N512S | 80 | 2.12195984 | 2.659928 | −114.1 | 0 | −2 | |
| P. hodgsonii | F8Y | 136 | 0.2337137 | −0.0661 | 3.7 | 0 | 0 |
| D407T | 76 | 0.63863865 | 0.801412 | 5 | 1 | −1 | |
| M449V | 129 | 0.05870691 | 1.199486 | −22.9 | 0 | 0 | |
| L467V | 34 | 0.18495316 | 0.683123 | −26.7 | 0 | 0 | |
| T509V | 52 | −0.2661298 | −0.22177 | 23.9 | 0 | −1 | |
| B. grunniens | Y440S | 108 | −0.3272085 | 0.142943 | −193.6 | 0 | 0 |
| S441P | 5 | 0.35454652 | −0.54554 | 112.7 | 0 | 1 | |
| I453V | 51 | 0.1775126 | −0.22684 | −26.7 | 0 | 0 | |
| F476L | 96 | 0.08862233 | 0.404623 | −23.2 | 0 | 0 |
Amino acid mutations of the COXI gene for C. hircus, P. hodgsonii, and B. grunniens, as compared with B. taurus. The influences of these mutations are predicted by the methods referred from Mirkovic et al. (31).
Fig. 2.
Alignment of amino acid sequences of the COXI gene using CLUSTAL W1.83. The species included were Bos taurus (P00396), Bubalus bubalis (YP_087084), Bos grunniens (YP_133786), Pantholops hodgsonii, Sus scrofa (NP_008636), Ovis aries(NP_008408), Muntiacus muntjak (AY225986), Canis familiaris (NP_008473), Equus caballus (NP_007162), Mus musculus (NP_904330), Capra hircus (NP_877405), Homo sapiens (NP_536845), Eschrichtius robustus (NP_944635), Ursus arctos (Q8SJI5), Ursus maritimus (Q8SJH3), Xenopus laevis (P00398), Shinisaurus crocodilurus (NC_005959), Gallus gallus (P18943), Anser albifrons (NP_777304), Brachydanio rerio (Q9MIY8), and Takifugu rubripes (NP_694917). Mutations of P. hodgsonii were highlighted as bold letters.
RNA genes
There are 22 tRNA genes identified in the P. hodgsonii mitochondrial genome, typical for mammalian mitochondrial genomes 6., 7., 8., 9.. Lengths of these tRNAs range from 65 to 74 bp. Some indels occur in the dihydrouridine and TѱC arms (Figure 1). The 12S rRNA and 16S rRNA genes are 957 bp and 1,566 bp in length, respectively.
Control region
Mitochondrial control region of P. hodgsonii is 1,067 bp in length, shorter than that of O. aries (1,180 bp), C. hircus (1,212 bp), domestic dog (1,270 bp), and domestic horse (1,192 bp), but longer than that of B. taurus (910 bp), B. indicus (913 bp), B. bubalis (910 bp), and B. grunniens (894 bp). By using the Tandem Repeats Finder (10), we found a 75-bp tandem repeats that vary among different species: four in O. aries (Ref. 6; another O. aries haplotype in the same study has three repeats), two in C. hircus, and two in P. hodgsonii. The 75-bp repeats appear at the same location for these three species, close to the 5′-end of the last prolinyl tRNA (Pro) gene (Figure 1). Sequence consensuses of these 75-bp repeats are very similar with identities of 86.7% between O. aries and P. hodgsonii, 74.0% between C. hircus and P. hodgsonii, and 76.6% between C. hircus and O. aries. In addition, P. hodgsonii has two additional 25-bp tandem repeats, which have not been found in Bos species. This repeat is inserted at the 3′-end of the P. hodgsonii control region, close to phenyalaninyl tRNA (Phe) gene (Figure 1).
The 75-bp repeat has a lower GC content (25%) than the average of the control region (39%), whereas the 25-bp repeat has slightly higher GC content (41%) than the average. Although we only found two 75-bp-long tandems in control region of the C. hircus mitochondrial genome, there is no loss of length in the C. hircus control region, compared to the same region of O. aries.
Phylogenetic analysis and time of divergence
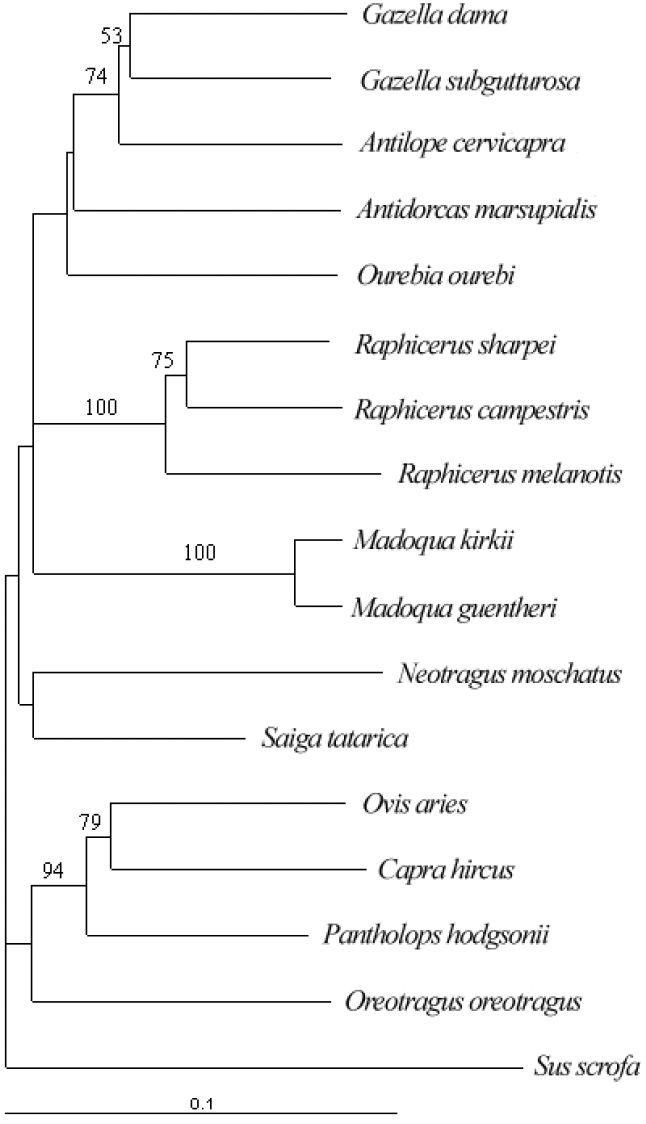
Complete cytochrome b of P. hodgsonii, O. aries, C. hircus, and other species of the Bovidae family were used to construct phylogenetic trees by using PAUP*(4b10) (11), with Giraffe camelopardalis as the outgroup. Our result indicates that P. hodgsonii is more related to O. aries, C. hircus, and Oreotragus oreotragus (klipspringer) (Figure 3), consistent with previously studies 12., 13.. Based on the phylogenetic tree and a substitution rate of 0.056 per site permillion years between two taxa (14), we estimated that P. hodgsonii and O. aries divided about 2.25 million years ago (Table 4).
Fig. 3.
Neighbor-joining tree based on the complete cytochrome b genes of some Bovidae species. The tree was constructed by using PAUP*(4b10), with parameters estimated by MODELTEST3.6 and with Giraffe camelopardalis as the outgroup. Number of bootstrap replication is 1000. Bootstrap values over 50% are shown on the branches.
Table 4.
Molecular Divergence and Estimated Divergence Time Between Native Mammals on the Tibetan Plateau and Their Lowland Relatives
| Species | Molecular divergence | Divergence time (million years before presence) |
|---|---|---|
| P. hodgsonii–C. hircus | 0.124 | 2.22 |
| P. hodgsonii–O. aries | 0.126 | 2.25 |
| O. aries–C. hircus | 0.124 | 2.21 |
| B. grunniens–B. taurus | 0.089 | 1.59 |
Based on complete sequences of mitochondrial cytochrome b and a substitution rate of 0.056 per site per million years between two mammalian taxa.
Positive selection analysis
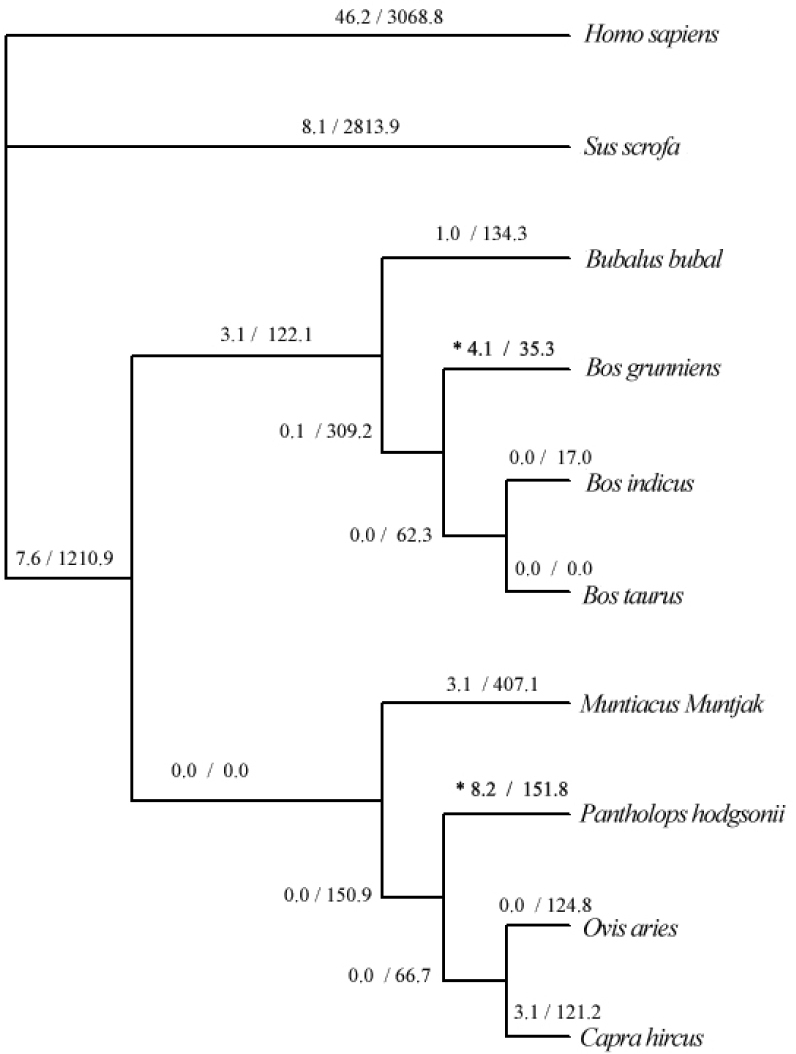
We used PAML (15) to analyze natural selection on mitochondrial genes of P. hodgsonii. The numbers of N (nonsynonymous substitutions) and S (synonymous substitutions) of each branch were calculated for eight genes that are more than 1,000 bp in length by codmel in PAML package. We found that N/S ratios of the COXI gene are significantly higher for the P. hodgsonii branch and the B. grunniens branch, compared with other branches (Fisher test P<0.001; Figure 4). The difference between N/S ratios of the B. grunniens branch and the P. hodgsonii branch is not significant (Fisher test P>0.1). These results suggested that the COXI gene has probably undergone positive selection in P. hodgsonii and B. grunniens. Despite the fact that COXI of P. hodgsonii has only three unique amino acid changes, it has other variation shared with one or more other species, which may collectively alter the function of the protein itself or interactions with other components in the mitochondrial respiration system. Experimentation and detailed structural analysis are of essence to pinpoint the relatedness of structure and function underscored by genetic changes. The sequence alignment of a highly variable region of COXI from selected species is shown in Figure 2.
Fig. 4.
The COXI gene was used to analyze N/S ratio of each branch within Artiodactyla mammals. Phylogeny was inferred with PAUP*(4b10). Numbers on the branches are mutation of nonsynonymous/synonymous for protein coding region of the COXI gene, as obtained with codeml in PAML package. * Fisher test P-value < 0.001. Take other branches (except B. grunniens and P. hodgsonii branches) as background for testing. The difference between the B. grunniens and P. hodgsonii branches is not significant (Fisher test P-value > 0.1).
Discussion
In the current study, we sequenced a complete mitochondrial genome from one P. hodgsonii individual, as the first attempt in a long-term research effort to understand the genetic basis of hypoxic adaptations of native fauna and flora on the Tibetan Plateau. Similar to other mammalian mitochondrial genomes, the P. hodgsonii mitochondrial genome contains 13 protein genes, 2 rRNA genes, 22 tRNA genes, and one control region. Its control region has two 75-bp tandem repeats near the last tRNA gene, whereas O. aries, C. hircus, and Bos species have three/four, two, and none, respectively. Although we only identified two 75-bp repeats in the control region of C. hircus, it is still possible that there might have been four units since C. hircus mitochondrial genome is very similar to the O. aries sequence in length and two of these four repeat units may occur early in time, resulted in poor homology due to mutations over a long decaying period. There are two additional 25-bp tandem repeats near the end of C. hircus’s control region, suggesting that short tandem repeats occur frequently among the mitochondrial genomes of O. aries, C. hircus, and P. hodgsonii.
Phylogenetic analysis on cytochrome b genes revealed that P. hodgsonii is more closely related with O. aries, C. hircus, and O. oreotragus, rather than other antelope species (the Antilopinae subfamily). Based on a substitution rate of 0.056 per site per million years between two mammalian taxa, we estimated that P. hodgsonii and O. aries divided about 2.25 million years ago. It was reported previously that climates around the Tibetan Plateau have undergone rapid changes at about 3.6−2.6 million years ago 16., 17.. Our results are in close agreement with the proposed climatic changes around the Tibetan Plateau.
To investigate whether the P. hodgsonii mitochondrial genes have been selected under the hypoxic environment of the plateau, we compared functional genes (CYTB, COXI, COXII, and COXIII) encoded in the mitochondrial genomes among P. hodgsonii and other distant relatives, including O. aries, C. hircus, and B. grunniens. In the COXI gene, we found that most of the mutations affecting amino acid sequences in P. hodgsonii and B. grunniens occur at sites between 400 and 500, whereas mutations in C. hircus occur at sites between 120 and 512. The region of 400 to 500 contains the transmembrane components X, XI, and XII, suggesting that nonsynonymous mutations unique to the two high altitude-adapted species may have functional implications. Although this process of functional prediction may not be very reliable, our results suggested to the likely targets for the next experimentation to verify the novel conjecture.
Evidence from N/S ratios shows that the COXI gene has more functional mutations in P. hodgsonii and B. grunniens compared with other mammals (Figure 4), providing further evidence that the COXI gene might have undergone positive selection among the native mammalian species of the plateau. The cytochrome c oxidase is the last step of the electron transport chain. It is consisted of 13 subunits, of which three subunits are encoded by the mitochondrial genome (COXI, II, and III), and ten subunits are encoded by the nuclear genome. Functional core of the enzyme complex is composed of subunits 1, 2, and 3 (18). Numerous studies have shown that some of the subunits of the COX gene have higher nonsynonymous substitution rate in primate than in other animals, such as COXI (19), COXII (20), and the nuclear coded COXIV (21) and COXVII (22). Several pieces of evidence suggest that structure and activity of cytochrome c oxidase may have adaptive changes during physiological hypoxia in mouse and rat cells (23). Expression of the mitochondrial genome encoded subunit COXI can decrease due to hypoxia while the enzyme efficiency remained. It suggested that expression of the COXI gene was regulated by the oxygen content. In addition, significant higher expression of COXI mRNA was observed in mammalian tissues, such as kidney and heart, during hibernation, and the change was not found in euthermic animals 24., 25..
Since P. hodgsonii and B. grunniens are both well-adapted to the same environment, the Tibetan Plateau, natural selection may have resulted in similar genetic signatures in their genomes, including the nuclear and mitochondrial genomes. Similar seemingly function-associated mutations and dN/dS ratios at COXI of P. hodgsonii and B. grunniens provided useful clues for further studies and functional confirmations on the role of mtDNA-encoded COX subunits on adaptation of native mammals to the unique Tibetan Plateau.
Materials and Methods
DNA extraction and sequencing
Blood samples of P. hodgsonii individuals were collected from the Kekexili Natural Reservation in Qinghai Province, China, in December 2004. Samples were stored at 4°C for a few days before whole genomic DNA was extracted from the whole blood with standard salt-extraction method.
PCR primers and sequencing primers were designed based on a sequence of O. aries mitochondrial genome (NC_001941; ref. 6). PCR reactions were conducted on a PTC-200 thermal cycler with the following conditions: an initial denaturation step of 95°C (3 min) followed by 34 cycles of 95°C (30 s), 58°C/56°C (30 s), and 72°C (90 s) followed by 72°C for 10 min. PCR products were purified by Montage PCR Cleanup Kit (Millipore, Billerica, USA) and sequenced with ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, USA).
Sequence analysis
Base calling was performed with Phred (26) at the lowest Phred quality values control of 20. The sequences were assembled with Phrap (http://www.phrap.org) in a default setting. Sequence contigs were further finished, compared, and annotated in referencing to that of the O. aries sequence (NC_001941). Genes of tRNA were defined with tRNAscan-SE 1.2 (http://www.genetics.wustl.edu/eddy/tRNAscan-SE/). Comparative analysis was performed by using BLAST (27) and CLUSTAL W1.83 (28). Tandem repeats were defined with the program Tandem Repeats Finder (10).
Phylogenetic analysis
Complete sequences of cytochrome b genes from P. hodgsonii, O. aries, C. hircus, and other selected species of the Bovidae family were used to construct phylogenetic trees, with G. camelopardalis as an outgroup. The sequences used were from P. hodgsonii, O. oreotragus (AF022052), Antilope cervicapra (AF022058), Ourebia ourebi (AF320574), Neotragus moschatu (AF022069), Madoqua kirki (AF022070), Madoqua guentheri (AF022071), Saiga tatarica (AF064487), Raphicerus sharpei (AF022050), Gazella dama (AF025954), Gazella subgutturosa (AF036282), Raphicerus campestris (AF022068), Raphicerus melanotis (AF022053), Antidorcas marsupialis (AF022054), O. aries (NC_001941), C. hircus (NC_005044), Bubalus bubal (NC_006295), B. grunniens (NC_006380), B. indicus (NC_005971), B. taurus (NC_006853), and G. camelopardalis (AB001612).
Sequences were aligned with CLUSTAL W1.83 in default options. Evolutionary models and parameters of the aligned sequences were estimated by MODELTEST3.6 (29). Phylogenetic trees were constructed with the neighbor joining arithmetic method using PAUP*(4b10). Bootstrap analysis of 1,000 replicates was performed to estimate robustness of the tree.
Analysis of positive selection
Eight protein coding genes with the length of over 1,000 bp were used to calculate N/S in each branch by using PAML, and phylogenetic trees were constructed with PAUP*(4b10). The F3X4 model for codon frequency and free ωratio model of oratio assumption was performed with the genetic codon of mammalian mitochondrial, and the other parameters were used as default. The DNA sequences were aligned as protein sequences. The following sequences were also included in the analysis from Homo sapiens (X93334), Sus scrofa (NC_000845), Bubalus bubal (NC_006295), B. grunniens (NC_006380), B. indicus (NC_005971), B. taurus (NC_006853), Muntiacus muntjak (AY225986), O. aries (NC_001941), C. hircus (NC_005044), and P. hodgsonii.
Acknowledgements
We are grateful to Dr. Ming-Sheng Yu of the Qinghai Academy of Animal and Veterinary Science, Qinghai University, staff of the Kekexili Natural Reservation, and students of Qinghai University Medical School for sample collection. Xiao-Mei Tong, Yan Guo, Jun Li, and staff of the Beijing Genomics Institute helped through molecular experiment and data analysis. Dr. Wen Wang provided invaluable comments for data analysis and the manuscript. This work was supported by Chinese Academy of Sciences (grant to Jun Yu, No. KSCX2-SW-331), National Natural Science Foundation of China (grant to Ri-Li Ge, No. 30393133), and Natural Research Foundation of Qinghai (grant to Ri-Li Ge, No. 2003-N-120).
Contributor Information
Xiao-Guang Zheng, Email: zhengxg@genomics.org.cn.
Ri-Li Ge, Email: geriligao@hotmail.com.
References
- 1.Nowak R.M. Walkers Mammals of the World. Sixth Edition. The Johns Hopkins University Press; Baltimore, USA: 1999. [Google Scholar]
- 2.Boore J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blier P.U. Natural selection and the evolution of mtDNA-encoded peptides: evidence for intergenomic co-adaptation. Trends Genet. 2001;17:400–406. doi: 10.1016/s0168-9525(01)02338-1. [DOI] [PubMed] [Google Scholar]
- 4.Bannikova A.A. Molecular markers and modern phylogenetics of mammals. Zh. Obshch. Biol. 2004;65:278–305. [PubMed] [Google Scholar]
- 5.Ojala D. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- 6.Hiendleder S. The complete mitochondrial DNA sequence of the domestic sheep (Ovis aries) and comparison with the other major ovine haplotype. J. Mol. Evol. 1998;47:441–448. doi: 10.1007/pl00006401. [DOI] [PubMed] [Google Scholar]
- 7.Anderson S. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 8.Lin C.S. Complete nucleotide sequence of pig (Sus scrofa) mitochondrial genome and dating evolutionary divergence within Artiodactyla. Gene. 1999;236:107–114. doi: 10.1016/s0378-1119(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 9.Parma P. The complete nucleotide sequence of goat (Capra hircus) mitochondrial genome. Goat mitochondrial genome. DNA Seq. 2003;14:199–203. doi: 10.1080/1042517031000089487. [DOI] [PubMed] [Google Scholar]
- 10.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swofford D.L. Sinauer Associates, Inc., Publishers; Sunderland, USA: 2002. PAUP*: phylogenetic analysis using parsimony (and other methods). Version 4 beta 10. CD-ROM. [Google Scholar]
- 12.Hassanin A. Molecular systematics of the subfamily Caprinae (Artiodactyla, Bovidae) as determined from cytochrome b sequences. J. Mammal. Evol. 1998;5:217–236. [Google Scholar]
- 13.Matthee C.A., Robinson T.J. Cytochrome b phylogeny of the family Bovidae: resolution within the Alcelaphini, Antilopini, Neotragini, and Tragelaphini. Mol. Phylogenet. Evol. 1999;12:31–46. doi: 10.1006/mpev.1998.0573. [DOI] [PubMed] [Google Scholar]
- 14.Zheng X. Historical demography and genetic structure of sister species: deermice (Peromyscus) in the North American temperate rain forest. Mol. Ecol. 2003;12:711–724. doi: 10.1046/j.1365-294x.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 16.An Z. Evolution of Asian monsoons and phased uplift of the Himalaya-Tibetan plateau since Late Miocene times. Nature. 2001;411:62–66. doi: 10.1038/35075035. [DOI] [PubMed] [Google Scholar]
- 17.Li J.J. Late Cenozoic intensive uplift of Qinghai-Xizang Plateau and its impacts on environments in surrounding area. Quarter. Sci. 2001;21:381–391. [Google Scholar]
- 18.Cooper C.E. Cytochrome c oxidase: structure, function, and membrane topology of the polypeptide subunits. Biochem. Cell. Biol. 1991;69:586–607. doi: 10.1139/o91-089. [DOI] [PubMed] [Google Scholar]
- 19.Wu W. Molecular evolution of cytochrome c oxidase subunit I in primates: is there coevolution between mitochondrial and nuclear genomes? Mol. Phylogenet. Evol. 2000;17:294–304. doi: 10.1006/mpev.2000.0833. [DOI] [PubMed] [Google Scholar]
- 20.Adkins R.M., Honeycutt R.L. Evolution of the primate cytochrome c oxidase subunit II gene. J. Mol. Evol. 1994;38:215–231. doi: 10.1007/BF00176084. [DOI] [PubMed] [Google Scholar]
- 21.Wu W. Molecular evolution of cytochrome c oxidase subunit IV: evidence for positive selection in simian primates. J. Mol. Evol. 1997;44:477–491. doi: 10.1007/pl00006172. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt T.R. Molecular evolution of the COX7A gene family in primates. Mol. Biol. Evol. 1999;16:619–626. doi: 10.1093/oxfordjournals.molbev.a026144. [DOI] [PubMed] [Google Scholar]
- 23.Vijayasarathy C. Adaptive changes in the expression of nuclear and mitochondrial encoded subunits of cytochrome c oxidase and the catalytic activity during hypoxia. Eur. J. Biochem. 2003;270:871–879. doi: 10.1046/j.1432-1033.2003.03447.x. [DOI] [PubMed] [Google Scholar]
- 24.Hittel D.S., Storey K.B. Differential expression of mitochondria-encoded genes in a hibernating mammal. J. Exp. Biol. 2002;205:1625–1631. doi: 10.1242/jeb.205.11.1625. [DOI] [PubMed] [Google Scholar]
- 25.Carey H.V. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 26.Ewing B., Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- 27.Altschul S.F. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Thompson J.D. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posada D., Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 30.Bae J.S. The mitochondrial genome of the firefly, Pyrocoelia rufa: complete DNA sequence, genome organization, and phylogenetic analysis with other insects. Mol. Phylogenet. Evol. 2004;32:978–985. doi: 10.1016/j.ympev.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Mirkovic N. Structure-based assessment of missense mutations in human BRCA1: implications for breast and ovarian cancer predisposition. Cancer Res. 2004;64:3790–3797. doi: 10.1158/0008-5472.CAN-03-3009. [DOI] [PubMed] [Google Scholar]