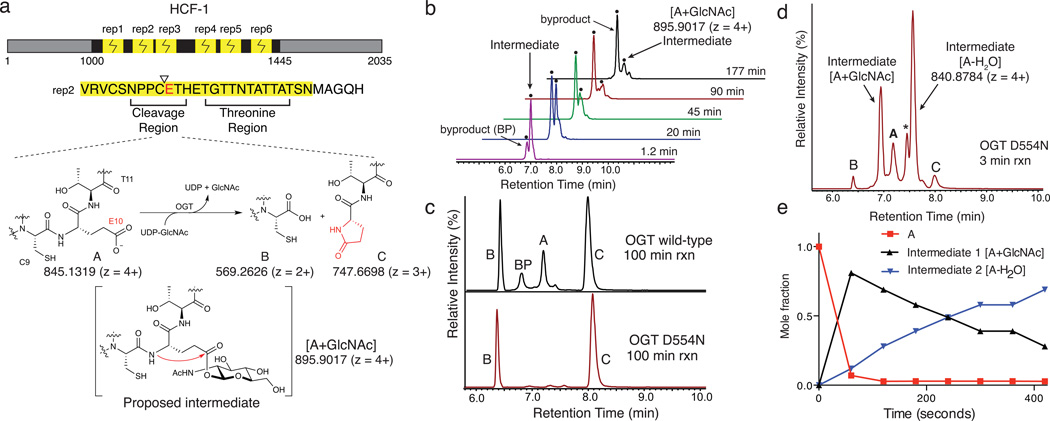
Figure 1.
Two intermediates form during OGT-mediated cleavage of an HCF-1 repeat. (a) HCF-1 contains six similar proteolytic repeats that are cleaved between residues C9 and E10, producing a C-terminal pyroglutamate product, C, that was proposed to form via the intermediacy of a glycosyl ester.12 (b) LC traces showing EICs for m/z 895.9017 ± 5 ppm, corresponding to A+GlcNAc, from the time course shown in Supplementary Figure 1. The y-axis scaling is such that the tallest peak is 100% (axis not shown). Two peaks having the same exact mass were observed, one of which is a reaction intermediate while the other is a byproduct glycopeptide. (c) EICs for cleavage reactions of HCF-short with wild-type OGT (top) and OGTD554N (bottom) showing A, B, C and [A+GlcNAc]. (d) An LC trace showing a 3 minute time point for cleavage of HCF-short using OGTD554N. Peaks for A, B, C, the [A+GlcNAc] intermediate, and another intermediate, [A-H2O], are shown. The ‘*’ denotes a second peak having the same m/z as the [A-H2O] species. The full time course is shown in Supplementary Figure 5. (e) The temporal sequence of events was established from a time course of cleavage using a large excess of OGTD554N. Mole fractions of A, [A+GlcNAc] and [A-H2O], were plotted as a function of time. The data was truncated at 420 seconds, such that products represent a negligible mole fraction. Mole fractions were determined based on the maximal signal for each species, normalized over all species at that time point.