Figure 5.
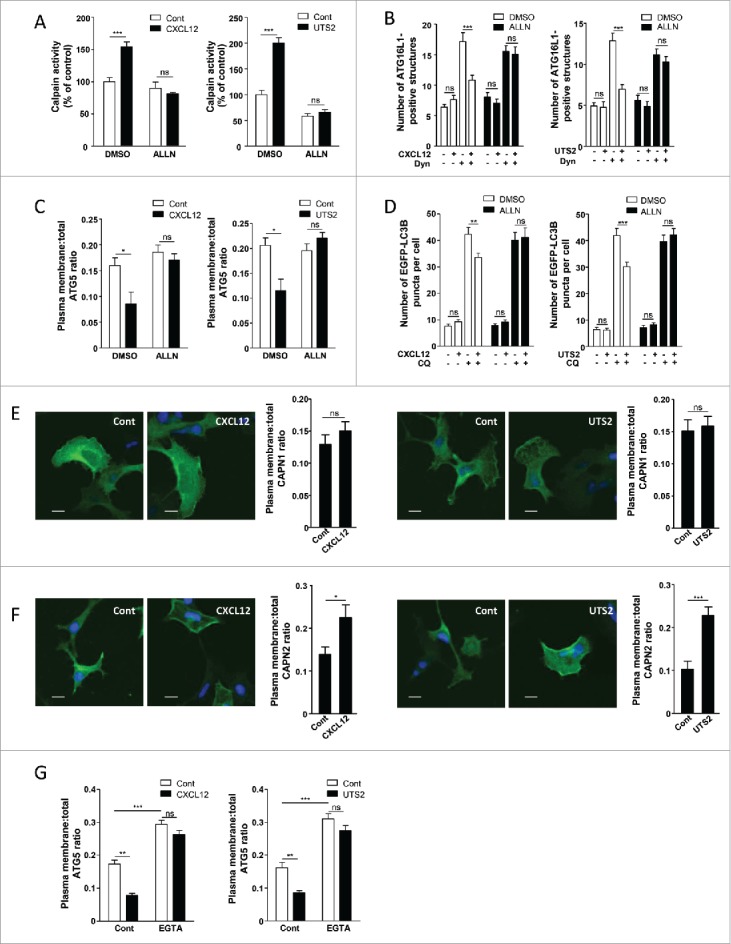
The anti-autophagic effects of chemotactic GPCRs depend on CAPN activation. (A) HEK-293 cells expressing CXCR4 (left panel) or UTS2R (right panel) were treated (1 h) with the respective ligands (CXCL12, 10−8 M; UTS2, 10−9 M), with or without ALLN (10−6 M), as indicated. For each group, CAPN activity was measured using the BOC-LM-CMAC fluorescence assay. Data represent means ± SEM from 20 photographic fields per group. (B) HEK-293 cells expressing CXCR4 (left panel) or UTS2R (right panel) were starved in HBSS for 1 h in the absence or presence of Dynasore (Dyn; 10−4 M), ALLN (10−6 M) and CXCL12 (10−8 M, left panel) or UTS2 (10−9 M, right panel) as indicated. Cells were fixed and labeled with an anti-ATG16L1 antibody. For each photographic field, the number of ATG16L1-positive structures was quantified and normalized to the number of nuclei. Data represent means ± SEM from 15 photographic fields per group. (C) HEK-293 cells expressing MYC-ATG5 together with CXCR4 (left panel) or UTS2R (right panel) were placed in DMEM without serum for 1 h with the respective ligands (CXCL12, 10−8 M; UTS2, 10−9 M), with or without ALLN (10−6 M), as indicated. Cells were fixed and labeled with an anti-MYC antibody. For each photographic field, the fraction of MYC-ATG5 protein localized at the plasma membrane was evaluated. Data represent means ± SEM from 15 photographic fields per group. (D) HEK-293 cells expressing EGFP-LC3B together with CXCR4 (left panel) or UTS2R (right panel) were placed in DMEM without serum for 6 h, with or without chloroquine (CQ, 5 × 10−5 M), ALLN (10−6 M) and CXCL12 (10−8 M, left panel) or UTS2 (10−9 M, right panel) as indicated. Cells were then fixed and the number of EGFP-LC3B fluorescent dots per cell was quantified in confocal images. Data represent means ± SEM, from at least 100 cells per group. (E) HEK-293 cells expressing Flag-CAPN1 together with CXCR4 (left panels) or UTS2R (right panels) were placed in DMEM without serum for 1 h with the respective ligands (CXCL12, 10−8 M; UTS2, 10−9 M). Cells were fixed and labeled with an anti-Flag antibody. For each photographic field, the fraction of Flag-CAPN1 protein localized at the plasma membrane was evaluated. Data represent means ± SEM from 15 photographic fields per group. Scale bars: 10 µm. (F) HEK-293 cells expressing Flag-CAPN2 together with CXCR4 (left panels) or UTS2R (right panels) were placed in DMEM without serum for 1 h with the respective ligands (CXCL12, 10−8 M; UTS2, 10−9 M). Cells were fixed and labeled with an anti-Flag antibody. For each photographic field, the fraction of Flag-CAPN2 protein localized at the plasma membrane was evaluated. Data represent means ± SEM from 15 photographic fields per group. Scale bars: 10 µm. (G) HEK-293 cells expressing MYC-ATG5 together with CXCR4 (left panel) or UTS2R (right panel) were placed in DMEM without serum for 1 h with the respective ligands (CXCL12, 10−8 M; UTS2, 10−9 M), with or without EGTA (5 × 10−3 M), as indicated. Cells were fixed and the fraction of MYC-ATG5 protein localized at the plasma membrane was evaluated as in (C). Statistical significance was evaluated using a Mann and Whitney test (A, B, C, E, F, G) or an unpaired t test (D). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not statistically different.
