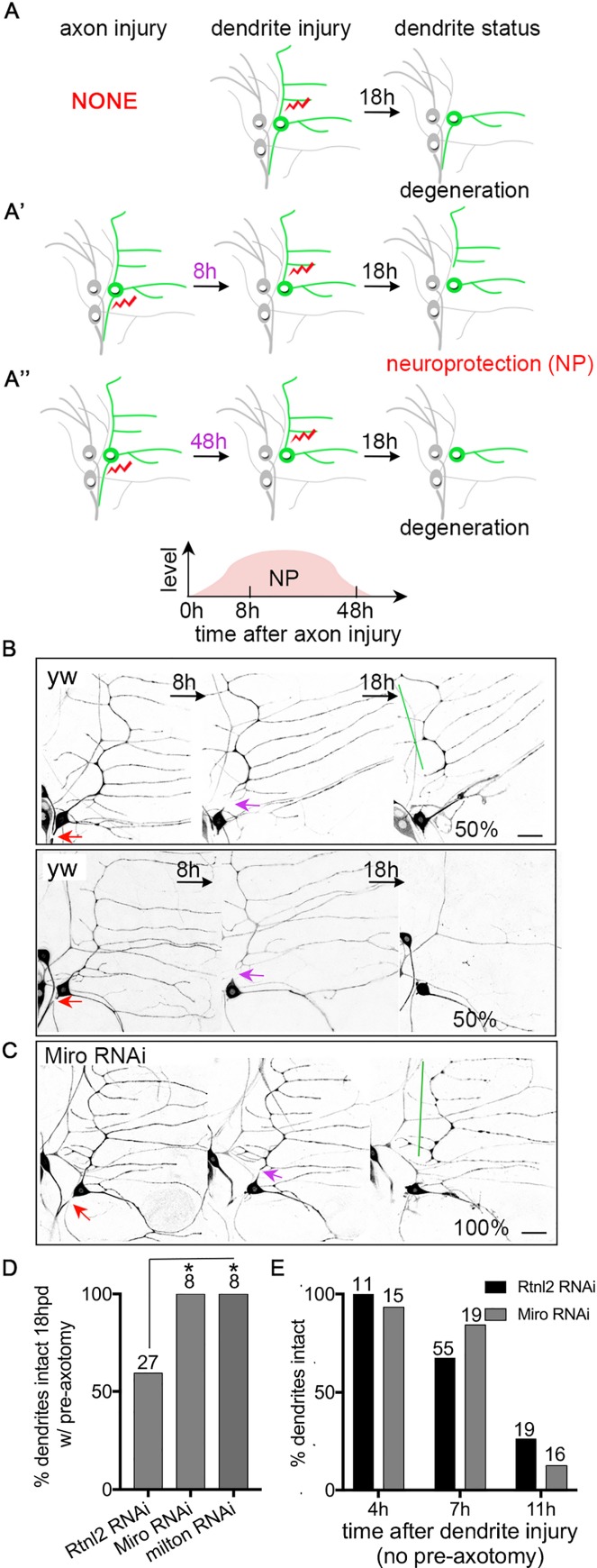
Fig 1. Reducing mitochondria in dendrites increases axotomy-induced neuroprotection.
(A-A”) A schematic of the axotomy-induced neuroprotection/NP assay is shown. (A) Without pre-axon injury, dendrites degenerate within 18h after injury. (A’) An axon injury 8h prior to dendrite injury induces NP so that dendrite degeneration is delayed; this timeline is the standard one used to assay NP throughout. (A”) When 48h elapses between the axon injury and dendrite severing, very little NP is observed and most dendrites are gone 18h after they are removed. Laser-induced injury is indicated by red lightning bolts. The ddaE neuron is drawn in green and other neurons labeled by 221-Gal4, which was used in most experiments to drive expression, are drawn in grey. (B and C) The NP assay as illustrated in Fig 1A’ was performed in wide-type (yw indicates control neurons that do not express an RNAi hairpin, and Rtnl2 indicates neurons that express a control RNAi hairpin) and Miro RNAi neurons. Neurons were labeled with EB1-GFP under the control of 221-Gal4. In control conditions half the neurons have a dendrite that remains at 18h, and (top row) and half have a fully degenerated dendrite (bottom row in B). Red arrows are the site of axon injury; purple arrows mark the site of dendrite injury. Green lines indicate stabilized dendrites. The scale bar is 20 μm. (D) Quantification of NP is shown. The number of neurons analyzed for each genotype is indicated above the bars. A Fisher’s exact test was used to determine statistical significance. Rtnl2 RNAi was used as a control as it targets a non-essential gene for which we have never observed phenotypes. * p<0.05. (E) Dendrites in ddaE neurons expressing EB1-GFP and control or Miro RNA hairpins were severed without prior axon injury. The presence of intact dendrites (no breaks in continuity) was scored at 4h, 7h and 11h after dendrites were severed. The numbers above the bars are the numbers of cells analyzed; one cell per animal.