Figure 3.
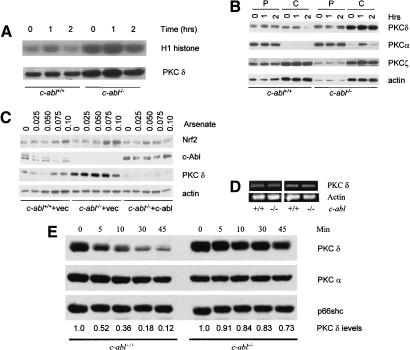
PKC δ expression was elevated in c-abl-/- osteoblasts. (A) Activation of PKC δ by arsenate. Mutant and control osteoblasts were treated with 0.10 mM arsenate for different periods of time, PKC δ was immunoprecipitated with an anti-PKC δ antibody, and in vitro kinase assay was performed using H1 histone as a substrate. (B) Expression of PKC δ in c-Abl-deficient and normal osteoblasts. Cell lysate was fractionated into cytosol and particulate portions and equal amounts were analyzed by Western blot using a monoclonal anti-PKC δ antibody. The blot was also probed with antibodies against other isoforms of the PKC family. (P) Particulate fraction; (C) cytosol fraction. (C) Reconstitution of c-Abl by a retroviral vector down-regulated the level of PKC δ in c-Abl-deficient cells and restored its normal accumulation of Nrf2. (D) The mRNA levels for PKC δ were normal in c-abl-/- osteoblasts. Total RNA was isolated from mutant and control osteoblast cultures and was used for RT–PCR with actin as a control. (E) Degradation of PKC δ induced by PMA was slower in c-abl-/- osteoblasts. Wild-type and c-abl-/- osteoblasts were treated with 500-nM PMA for different periods of time and the protein levels of PKC δ were determined by Western blot. The blot was stripped and reprobed with anti-PKC α and Shc, respectively, which served as a loading control. The levels of PKC δ were determined by densitometry and compared with the basal levels, which were set at 1.0.