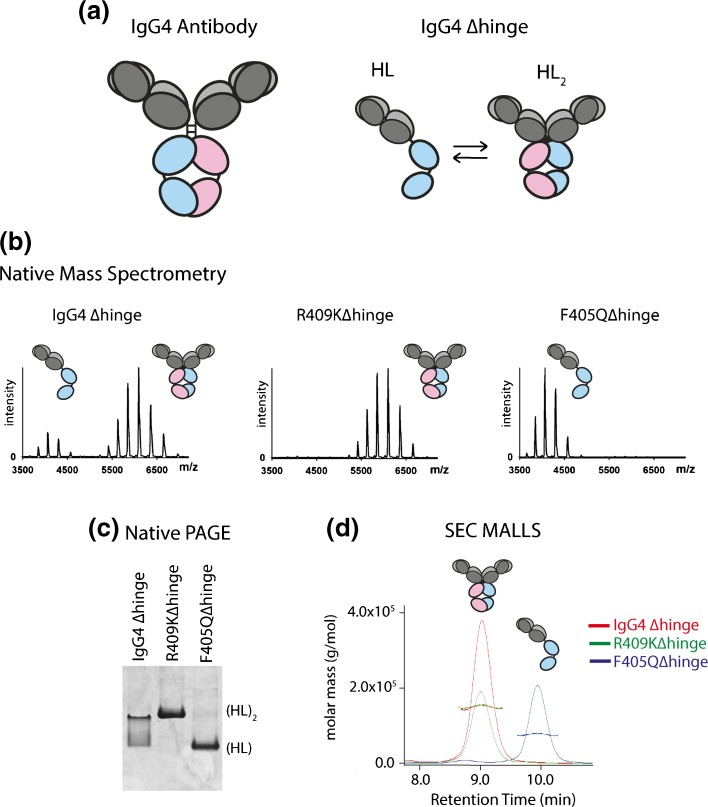
Figure 3.
Native MS compares well with alternative methods to probe quaternary structures. (a) Cartoon of the wild-type IgG4 antibody and an IgG4 antibody whereby the hinge region is deleted (IgG4Δhinge). IgG4Δhinge exists in solution in equilibrium between its monomeric (HL) and dimeric (HL2) state. (b) Native MS of three HL mutants; IgG4Δhinge, R409KΔhinge, and F405QΔhinge. Two charge state distributions are observed for IgG4Δhinge corresponding to its monomeric and dimeric state, well separated in m/z space. In contrast, predominantly the monomeric and dimeric states were observed for the IgG4Δhinge variants F405Q and R409K, respectively. (c) Native PAGE of IgG4Δhinge, R409KΔhinge, and F405QΔhinge, showing different band migrations corresponding to the oligomeric status (i.e., monomeric/dimeric) of the variants in solution. (d) Size exclusion chromatography (SEC) multi-angle laser light scattering (MALLS) of IgG4Δhinge (red), R409KΔhinge (green), and F405QΔhinge (blue), showing the difference in retention times/molar masses measured reflecting their either monomeric, dimeric, or mixed status in solution [81]. The figure is adapted from [81]