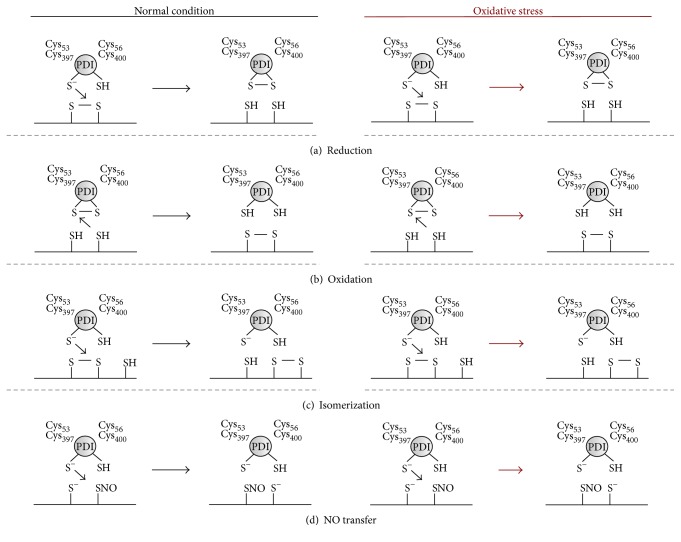
Figure 1.
Reactions catalyzed by PDI and possible unbalance in oxidative stress. In (a), PDI catalyzes reduction of a disulfide bond to a dithiol through an attack from its thiolate anion located in Cys53 or Cys397, whereas in (b) shows PDI oxidizing a dithiol into a disulfide bond. In (c), PDI isomerizes a disulfide bond in the same molecule. These reactions are expected to be increased in OxS, since the reduction of disulfide bonds has been shown to promote platelet aggregation and isomerization is an essential step towards α 2b β 3 activation [18]. In (d), PDI reacts with NO to promote transnitrosation, shifting NO from one molecule to another or within the same molecule. It should be noted that PDI might also catalyze denitrosation, releasing NO from S-nitrosothiols. This reaction is expected to be decreased in oxidative stress mainly due to the decreased NO bioavailability.