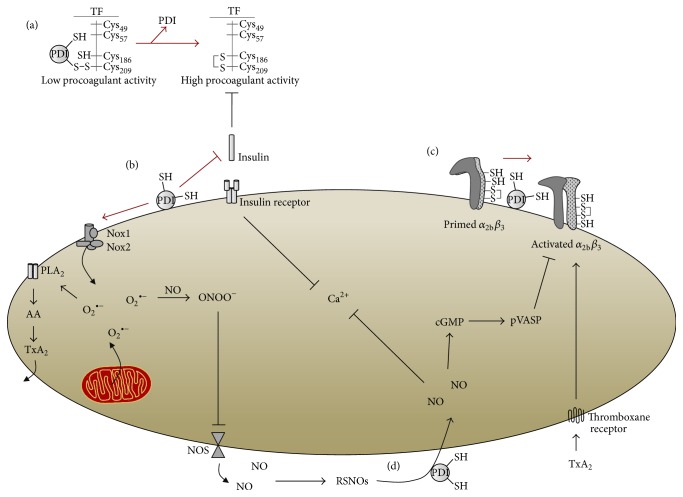
Figure 2.
PDI participates in mechanisms of platelet hyperactivation induced by metabolic syndrome. In (a), PDI promotes the procoagulant activity of tissue factor (TF) through the formation of a disulfide bond between TF Cys186 and Cys209. In (b), PDI inhibits insulin's action by reducing a disulfide bond that precipitates insulin's β-chain, preventing insulin's inhibitory activity upon TF and insulin's intracellular signaling in platelets. Also, PDI regulates Nox enzymes, promoting stronger generation of O2 ∙− that can either react with NO, forming peroxynitrite that will inhibit nitric oxide synthase (NOS) or induce thromboxane (e.g., A2) generation through phospholipase A2 (PLA2) and subsequent COX-derived arachidonic acid (AA) platelet metabolism. (c) PDI promotes the isomerization of a disulfide bond in α 2b β 3 integrin. Finally, in (d), PDI has a paradoxical effect in platelet aggregation, acting as a nitric oxide (NO) carrier and releaser through transnitrosation and denitrosation reactions of S-nitrosothiols (RSNOs). Arrows in red indicate overactivated mechanisms.