Figure 1. Molecular model of mouse Dishevelled 1 DEP domain structure with the lowest target function.
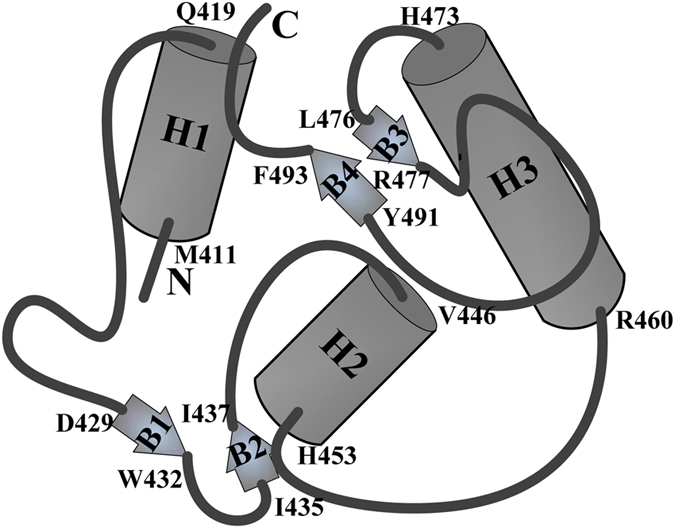
The diagram shows the residue range from the N-terminal 402aa to C-terminal 495aa, including three α helices(H1, H2, and H3) and four β sheets (B1–B4). The numbers in the figure indicate the beginning and end of the corresponding secondary structures. Many hydrophobic residues were situated in H1, H2, H3, B3, B4 to construct the hydrophobic core of DEP domain. Human Dishevelled 1 DEP domain possesses the same structure except for the amino acid position.
