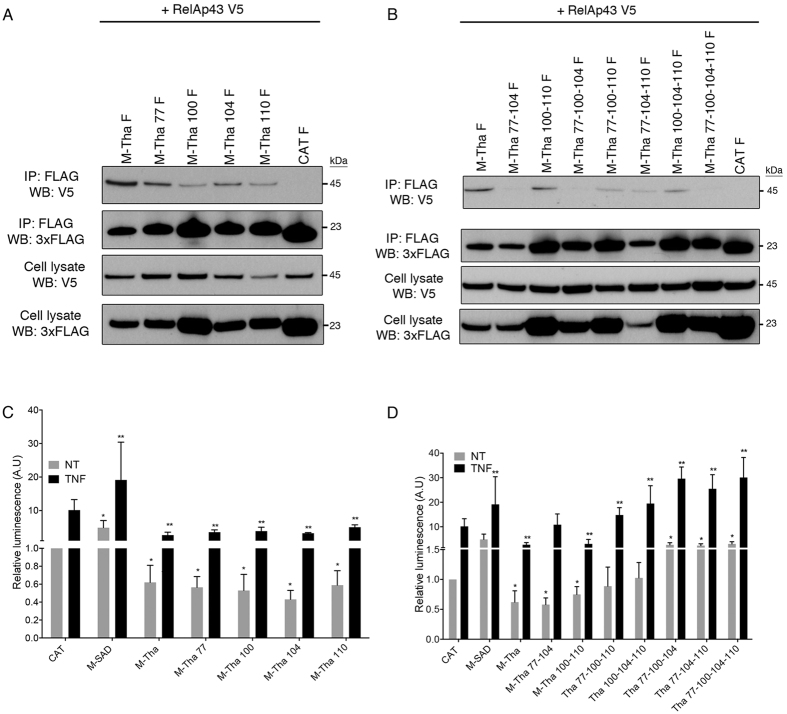
Figure 3. Residues at positions 77 and 104 are necessary for the interaction of M-Tha with RelAp43 and should be coupled with mutations D100A and/or M110L to restore a level of activation of the NF-κB reporter vector comparable to M-SAD.
(A and B) HeLa cells were used to co-express V5-tagged RelAp43 in combination with the indicated FLAG-tagged single (A) or multiple (B) mutants of M-Tha. After 24 h, cells were lysed, protein expression in cell lysates was controlled (cell lysate: WB V5; cell lysate: WB 3xFLAG on the Figure) and co-IP experiments were performed using anti-FLAG M2 beads. Interaction of RelAp43 with the different M constructs or CAT was visualized by western blot (IP: 3xFLAG; WB: V5 on the picture). The western blots depicted here are the results of a representative experiment (three repeats). (C) Modulation of NF-κB activation in 293T cells in the presence of single or double mutants of M-Tha compared to M-Tha, M-SAD or CAT. The NF-κB pathway was exogenously activated using 10 ng/mL TNF-α during 5 h (black bars) or left untreated (grey bars). The CAT control without the TNF treatment was used as the reference condition. (D) Modulation of NF-κB activation in 293T cells in the presence of triple or quadruple mutants of M-Tha compared to M-Tha, M-SAD or CAT. The NF-κB pathway was exogenously activated using 10 ng/mL TNF-α during 5 h (black bars) or left untreated (grey bars). The CAT control without the TNF treatment was used as the reference condition. Significant results (p < 0.05) in comparison to untreated (*) and TNF treated (**) CAT transfected cells were annotated.